Non-local means denoising of dynamic PET images
- PMID: 24339921
- PMCID: PMC3855264
- DOI: 10.1371/journal.pone.0081390
Non-local means denoising of dynamic PET images
Abstract
Objective: Dynamic positron emission tomography (PET), which reveals information about both the spatial distribution and temporal kinetics of a radiotracer, enables quantitative interpretation of PET data. Model-based interpretation of dynamic PET images by means of parametric fitting, however, is often a challenging task due to high levels of noise, thus necessitating a denoising step. The objective of this paper is to develop and characterize a denoising framework for dynamic PET based on non-local means (NLM).
Theory: NLM denoising computes weighted averages of voxel intensities assigning larger weights to voxels that are similar to a given voxel in terms of their local neighborhoods or patches. We introduce three key modifications to tailor the original NLM framework to dynamic PET. Firstly, we derive similarities from less noisy later time points in a typical PET acquisition to denoise the entire time series. Secondly, we use spatiotemporal patches for robust similarity computation. Finally, we use a spatially varying smoothing parameter based on a local variance approximation over each spatiotemporal patch.
Methods: To assess the performance of our denoising technique, we performed a realistic simulation on a dynamic digital phantom based on the Digimouse atlas. For experimental validation, we denoised [Formula: see text] PET images from a mouse study and a hepatocellular carcinoma patient study. We compared the performance of NLM denoising with four other denoising approaches - Gaussian filtering, PCA, HYPR, and conventional NLM based on spatial patches.
Results: The simulation study revealed significant improvement in bias-variance performance achieved using our NLM technique relative to all the other methods. The experimental data analysis revealed that our technique leads to clear improvement in contrast-to-noise ratio in Patlak parametric images generated from denoised preclinical and clinical dynamic images, indicating its ability to preserve image contrast and high intensity details while lowering the background noise variance.
Conflict of interest statement
Figures
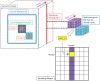
 and a voxel
and a voxel  , given by (3), is derived from spatiotemporal patches at the two voxels, composed of the local spatial neighborhood (denoted
, given by (3), is derived from spatiotemporal patches at the two voxels, composed of the local spatial neighborhood (denoted  for voxel
for voxel  ) and all time points beyond a temporal threshold
) and all time points beyond a temporal threshold  . The pairwise weights can be assembled into a symmetric matrix of similarities as shown.
. The pairwise weights can be assembled into a symmetric matrix of similarities as shown.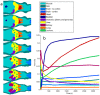


 was computed from the true, noisy, Gaussian-denoised, PCA-denoised, HYPR-denoised, NLM-S denoised, and NLM-ST denoised images of the dynamic Digimouse phantom. (b) Plots of overall percentage bias vs. percentage standard deviation for the Patlak parametric images computed from the noisy and denoised dynamic images.
was computed from the true, noisy, Gaussian-denoised, PCA-denoised, HYPR-denoised, NLM-S denoised, and NLM-ST denoised images of the dynamic Digimouse phantom. (b) Plots of overall percentage bias vs. percentage standard deviation for the Patlak parametric images computed from the noisy and denoised dynamic images.
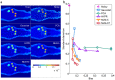
 was computed using noisy, Gaussian-denoised, PCA-denoised, HYPR-denoised, NLM-S denoised, and NLM-ST denoised images from an
was computed using noisy, Gaussian-denoised, PCA-denoised, HYPR-denoised, NLM-S denoised, and NLM-ST denoised images from an  PET mouse study. (b) The top row delineates the signal (red) and background (blue) ROIs used for evaluation. The middle row shows the percentage recovered signal in the hot regions for different denoising methods. The bottom row shows the CNR in the Patlak parametric images, measured as the ratio of the contrast between the signal and the background ROIs to the standard deviation in the background.
PET mouse study. (b) The top row delineates the signal (red) and background (blue) ROIs used for evaluation. The middle row shows the percentage recovered signal in the hot regions for different denoising methods. The bottom row shows the CNR in the Patlak parametric images, measured as the ratio of the contrast between the signal and the background ROIs to the standard deviation in the background.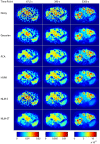

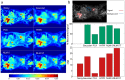
 was computed using noisy, Gaussian-denoised, PCA-denoised, HYPR-denoised, NLM-S denoised, and NLM-ST denoised images from an
was computed using noisy, Gaussian-denoised, PCA-denoised, HYPR-denoised, NLM-S denoised, and NLM-ST denoised images from an  PET scan of a patient with hepatocellular carcinoma. (b) The top row delineates the signal (lesions marked in red) and background (spleen marked in blue) ROIs used for evaluation. The middle row shows the percentage recovered signal in the hot lesions for different denoising methods. The bottom row shows the CNR in the Patlak parametric images, measured as the ratio of the contrast between the signal and the background ROIs to the standard deviation in the background.
PET scan of a patient with hepatocellular carcinoma. (b) The top row delineates the signal (lesions marked in red) and background (spleen marked in blue) ROIs used for evaluation. The middle row shows the percentage recovered signal in the hot lesions for different denoising methods. The bottom row shows the CNR in the Patlak parametric images, measured as the ratio of the contrast between the signal and the background ROIs to the standard deviation in the background.References
-
- Delbeke D (1999) Oncological applications of FDG PET imaging: brain tumors, colorectal cancer lymphoma and melanoma. J Nucl Med 40: 591–603. - PubMed
-
- Bergmann S, Fox K, Rand A, McElvany K, Welch M, et al. (1984) Quantification of regional myocardial blood flow in vivo with H15 2 O. Circulation 70: 724–733. - PubMed
-
- Farde L, Eriksson L, Blomquist G, Halldin C (1989) Kinetic analysis of central [11C] raclopride binding to D2-dopamine receptors studied by PET a comparison to the equilibrium analysis. J Cereb Blood Flow Metab 9: 696–708. - PubMed
-
- Huesman RH, Mazoyer BM (1987) Kinetic data analysis with a noisy input function. Phys Med Biol 32: 1569–1579. - PubMed
-
- Bertoldo A, Peltoniemi P, Oikonen V, Knuuti J, Nuutila P, et al. (2001) Kinetic modeling of [18F]FDG in skeletal muscle by PET: a four-compartment five-rate-constant model. Am J Physiol Endocrinol Metab 281: E524–E536. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

