Nuclear modifier MTO2 modulates the aminoglycoside-sensitivity of mitochondrial 15S rRNA C1477G mutation in Saccharomyces cerevisiae
- PMID: 24339937
- PMCID: PMC3858254
- DOI: 10.1371/journal.pone.0081490
Nuclear modifier MTO2 modulates the aminoglycoside-sensitivity of mitochondrial 15S rRNA C1477G mutation in Saccharomyces cerevisiae
Abstract
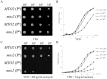
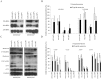
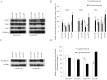
The phenotypic manifestations of mitochondrial DNA (mtDNA) mutations are modulated by mitochondrial DNA haplotypes, nuclear modifier genes and environmental factors. The yeast mitochondrial 15S rRNA C1477G (P(R) or P(R) 454) mutation corresponds to the human 12S rRNA C1494T and A1555G mutations, which are well known as primary factors for aminoglycoside-induced nonsyndromic deafness. Here we report that the deletion of the nuclear modifier gene MTO2 suppressed the aminoglycoside-sensitivity of mitochondrial 15S rRNA C1477G mutation in Saccharomyces cerevisiae. First, the strain with a single mtDNA C1477G mutation exhibited hypersensitivity to neomycin. Functional assays indicated that the steady-state transcription level of mitochondrial DNA, the mitochondrial respiratory rate, and the membrane potential decreased significantly after neomycin treatment. The impaired mitochondria could not produce sufficient energy to maintain cell viability. Second, when the mto2 null and the mitochondrial C1477G mutations co-existed (mto2(P(R))), the oxygen consumption rate in the double mutant decreased markedly compared to that of the control strains (MTO2(P(S)), mto2(P(S)) and MTO2(P(R))). The expression levels of the key glycolytic genes HXK2, PFK1 and PYK1 in the mto2(P(R)) strain were stimulated by neomycin and up-regulated by 89%, 112% and 55%, respectively. The enhanced glycolysis compensated for the respiratory energy deficits, and could be inhibited by the glycolytic enzyme inhibitor. Our findings in yeast will provide a new insight into the pathogenesis of human deafness.
Conflict of interest statement
Figures




References
-
- Ryan MT, Hoogenraad NJ (2007) Mitochondrial-nuclear communications. Annu Rev Biochem 76: 701–722. - PubMed
-
- Prezant TR, Agapian JV, Bohlman MC, Bu X, Oztas S, et al. (1993) Mitochondrial ribosomal RNA mutation associated with both antibiotic-induced and non-syndromic deafness. Nat Genet 4: 289–294. - PubMed
-
- Yuan H, Jiang S, Yang W, Guo W, Cao J, et al. (1999) [Screening for mitochondrial 1555(G) mutation in patients with aminoglycoside antibiotic-induced deafness]. Zhonghua Yi Xue Yi Chuan Xue Za Zhi 16: 141–144. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials

