Structural insights into the globular tails of the human type v myosins Myo5a, Myo5b, And Myo5c
- PMID: 24339992
- PMCID: PMC3858360
- DOI: 10.1371/journal.pone.0082065
Structural insights into the globular tails of the human type v myosins Myo5a, Myo5b, And Myo5c
Abstract
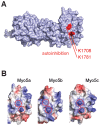
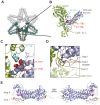
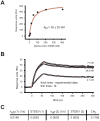
Vertebrate type V myosins (MyoV) Myo5a, Myo5b, and Myo5c mediate transport of several different cargoes. All MyoV paralogs bind to cargo complexes mainly by their C-terminal globular domains. In absence of cargo, the globular domain of Myo5a inhibits its motor domain. Here, we report low-resolution SAXS models for the globular domains from human Myo5a, Myo5b, and Myo5c, which suggest very similar overall shapes of all three paralogs. We determined the crystal structures of globular domains from Myo5a and Myo5b, and provide a homology model for human Myo5c. When we docked the Myo5a crystal structure into a previously published electron microscopy density of the autoinhibited full-length Myo5a, only one domain orientation resulted in a good fit. This structural arrangement suggests the participation of additional region of the globular domain in autoinhibition. Quantification of the interaction of the Myo5a globular domain with its motor complex revealed a tight binding with dissociation half-life in the order of minutes, suggesting a rather slow transition between the active and inactive states.
Conflict of interest statement
Figures


Similar articles
-
Identification of the Isoform-specific Interactions between the Tail and the Head of Class V Myosin.J Biol Chem. 2016 Apr 8;291(15):8241-50. doi: 10.1074/jbc.M115.693762. Epub 2016 Feb 24. J Biol Chem. 2016. PMID: 26912658 Free PMC article.
-
Purification, crystallization and preliminary crystallographic analysis of the globular domain of the human type V myosin Myo5a.Acta Crystallogr Sect F Struct Biol Cryst Commun. 2013 Nov;69(Pt 11):1220-3. doi: 10.1107/S1744309113025578. Epub 2013 Oct 17. Acta Crystallogr Sect F Struct Biol Cryst Commun. 2013. PMID: 24192353 Free PMC article.
-
Organelle targeting of myosin XI is mediated by two globular tail subdomains with separate cargo binding sites.J Biol Chem. 2007 Jul 13;282(28):20593-602. doi: 10.1074/jbc.M700645200. Epub 2007 May 11. J Biol Chem. 2007. PMID: 17500056
-
Myosin V from head to tail.Cell Mol Life Sci. 2008 May;65(9):1378-89. doi: 10.1007/s00018-008-7507-6. Cell Mol Life Sci. 2008. PMID: 18239852 Free PMC article. Review.
-
The tail that wags the dog: the globular tail domain defines the function of myosin V/XI.Traffic. 2008 Mar;9(3):290-8. doi: 10.1111/j.1600-0854.2007.00687.x. Epub 2007 Dec 9. Traffic. 2008. PMID: 18088322 Review.
Cited by
-
Melanophilin Stimulates Myosin-5a Motor Function by Allosterically Inhibiting the Interaction between the Head and Tail of Myosin-5a.Sci Rep. 2015 Jun 3;5:10874. doi: 10.1038/srep10874. Sci Rep. 2015. PMID: 26039755 Free PMC article.
-
Integrated miRNA-mRNA transcriptomic analysis reveals epigenetic-mediated embryonic muscle growth differences between Wuzhishan and Landrace pigs1.J Anim Sci. 2019 Apr 29;97(5):1967-1978. doi: 10.1093/jas/skz091. J Anim Sci. 2019. PMID: 31222274 Free PMC article.
-
Regulation of class V myosin.Cell Mol Life Sci. 2018 Jan;75(2):261-273. doi: 10.1007/s00018-017-2599-5. Epub 2017 Jul 20. Cell Mol Life Sci. 2018. PMID: 28730277 Free PMC article. Review.
-
The Globular Tail Domain of Myosin-5a Functions as a Dimer in Regulating the Motor Activity.J Biol Chem. 2016 Jun 24;291(26):13571-9. doi: 10.1074/jbc.M116.724328. Epub 2016 Apr 28. J Biol Chem. 2016. PMID: 27129208 Free PMC article.
-
Identification of the Isoform-specific Interactions between the Tail and the Head of Class V Myosin.J Biol Chem. 2016 Apr 8;291(15):8241-50. doi: 10.1074/jbc.M115.693762. Epub 2016 Feb 24. J Biol Chem. 2016. PMID: 26912658 Free PMC article.
References
-
- Hammer JA 3rd, Sellers JR (2012) Walking to work: roles for class V myosins as cargo transporters. Nat Rev Mol Cell Biol 13: 13–26. - PubMed
-
- Vale RD (2003) The molecular motor toolbox for intracellular transport. Cell 112: 467–480. - PubMed
-
- Desnos C, Huet S, Darchen F (2007) ‘Should I stay or should I go?’: myosin V function in organelle trafficking. Biol Cell 99: 411–423. - PubMed
-
- Rodriguez OC, Cheney RE (2002) Human myosin-Vc is a novel class V myosin expressed in epithelial cells. J Cell Sci 115: 991–1004. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources

