Nogo-receptor 1 deficiency has no influence on immune cell repertoire or function during experimental autoimmune encephalomyelitis
- PMID: 24339996
- PMCID: PMC3855334
- DOI: 10.1371/journal.pone.0082101
Nogo-receptor 1 deficiency has no influence on immune cell repertoire or function during experimental autoimmune encephalomyelitis
Abstract
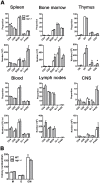
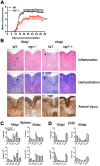
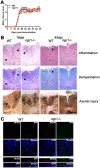
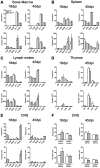
The potential role of Nogo-66 Receptor 1 (NgR1) on immune cell phenotypes and their activation during neuroinflammatory diseases such as multiple sclerosis (MS) and its animal model, experimental autoimmune encephalomyelitis (EAE), is unclear. To further understand the function of this receptor on haematopoietically-derived cells, phenotypic and functional analyses were performed using NgR1-deficient (ngr1-/-) animals. Flow cytometry-based phenotypic analyses performed on blood, spleen, thymus, lymph nodes, bone marrow and central nervous-system (CNS)-infiltrating blood cells revealed no immunological defects in naïve ngr1-/- animals versus wild-type littermate (WTLM) controls. EAE was induced by either recombinant myelin oligodendrocyte glycoprotein (rMOG), a model in which B cells are considered to contribute pathogenically, or by MOG35-55 peptide, a B cell-independent model. We have demonstrated that in ngr1-/- mice injected with MOG35-55, a significant reduction in the severity of EAE correlated with reduced axonal damage present in the spinal cord when compared to their WTLM controls. However, despite a reduction in axonal damage observed in the CNS of ngr1-/- mice at the chronic stage of disease, no clinical differences could be attributed to a specific genotype when rMOG was used as the encephalitogen. Following MOG35-55-induction of EAE, we could not derive any major changes to the immune cell populations analyzed between ngr1-/- and WTLM mice. Collectively, these data demonstrate that NgR1 has little if any effects on the repertoire of immune cells, their activation and trafficking to the CNS.
Conflict of interest statement
Figures
Similar articles
-
Limiting multiple sclerosis related axonopathy by blocking Nogo receptor and CRMP-2 phosphorylation.Brain. 2012 Jun;135(Pt 6):1794-818. doi: 10.1093/brain/aws100. Epub 2012 Apr 28. Brain. 2012. PMID: 22544872 Free PMC article.
-
Nogo-receptors NgR1 and NgR2 do not mediate regulation of CD4 T helper responses and CNS repair in experimental autoimmune encephalomyelitis.PLoS One. 2011;6(11):e26341. doi: 10.1371/journal.pone.0026341. Epub 2011 Nov 11. PLoS One. 2011. PMID: 22096481 Free PMC article.
-
B-cells expressing NgR1 and NgR3 are localized to EAE-induced inflammatory infiltrates and are stimulated by BAFF.Sci Rep. 2021 Feb 3;11(1):2890. doi: 10.1038/s41598-021-82346-6. Sci Rep. 2021. PMID: 33536561 Free PMC article.
-
Autoimmune pathogenesis of multiple sclerosis: role of autoreactive T lymphocytes and new immunotherapeutic strategies.Crit Rev Immunol. 1997;17(1):33-75. doi: 10.1615/critrevimmunol.v17.i1.20. Crit Rev Immunol. 1997. PMID: 9034723 Review.
-
Role of Th17 cells in the pathogenesis of CNS inflammatory demyelination.J Neurol Sci. 2013 Oct 15;333(1-2):76-87. doi: 10.1016/j.jns.2013.03.002. Epub 2013 Apr 8. J Neurol Sci. 2013. PMID: 23578791 Free PMC article. Review.
Cited by
-
Nogo receptor complex expression dynamics in the inflammatory foci of central nervous system experimental autoimmune demyelination.J Neuroinflammation. 2016 Oct 11;13(1):265. doi: 10.1186/s12974-016-0730-4. J Neuroinflammation. 2016. PMID: 27724971 Free PMC article.
-
Regulatory T Cell Dysfunction Acquiesces to BTLA+ Regulatory B Cells Subsequent to Oral Intervention in Experimental Autoimmune Encephalomyelitis.J Immunol. 2016 Jun 15;196(12):5036-46. doi: 10.4049/jimmunol.1501973. Epub 2016 May 18. J Immunol. 2016. PMID: 27194787 Free PMC article.
-
p75NTR and TROY: Uncharted Roles of Nogo Receptor Complex in Experimental Autoimmune Encephalomyelitis.Mol Neurobiol. 2018 Aug;55(8):6329-6336. doi: 10.1007/s12035-017-0841-7. Epub 2018 Jan 2. Mol Neurobiol. 2018. PMID: 29294247 Review.
-
Immune Repertoires in Various Dermatologic and Autoimmune Diseases.Genes (Basel). 2024 Dec 11;15(12):1591. doi: 10.3390/genes15121591. Genes (Basel). 2024. PMID: 39766858 Free PMC article. Review.
References
-
- Trapp BD, Ransohoff R, Rudick R (1999) Axonal pathology in multiple sclerosis: relationship to neurologic disability. Curr Opin Neurol 12: 295–302. - PubMed
-
- McQualter JL, Bernard CC (2007) Multiple sclerosis: a battle between destruction and repair. J Neurochem 100: 295–306. - PubMed
-
- Baranzini SE, Nickles D (2012) Genetics of multiple sclerosis: swimming in an ocean of data. Curr Opin Neurol 25: 239–245. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases

