Methylene blue modulates transendothelial migration of peripheral blood cells
- PMID: 24340007
- PMCID: PMC3858277
- DOI: 10.1371/journal.pone.0082214
Methylene blue modulates transendothelial migration of peripheral blood cells
Abstract
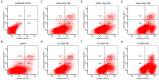
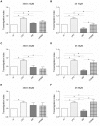
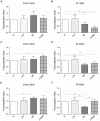
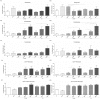
Vasoplegia is a severe complication after cardiac surgery. Within the last years the administration of nitric oxide synthase inhibitor methylene blue (MB) became a new therapeutic strategy. Our aim was to investigate the role of MB on transendothelial migration of circulating blood cells, the potential role of cyclic cGMP, eNOS and iNOS in this process, and the influence of MB on endothelial cell apoptosis. Human vascular endothelial cells (HuMEC-1) were treated for 30 minutes or 2 hours with different concentrations of MB. Inflammation was mimicked by LPS stimulation prior and after MB. Transmigration of PBMCs and T-Lymphocytes through the treated endothelial cells was investigated. The influence of MB upon the different subsets of PBMCs (Granulocytes, T- and B-Lymphocytes, and Monocytes) was assessed after transmigration by means of flow-cytometry. The effect of MB on cell apoptosis was evaluated using Annexin-V and Propidium Iodide stainings. Analyses of the expression of cyclic cGMP, eNOS and iNOS were performed by means of RT-PCR and Western Blot. Results were analyzed using unpaired Students T-test. Analysis of endothelial cell apoptosis by MB indicated a dose-dependent increase of apoptotic cells. We observed time- and dose-dependent effects of MB on transendothelial migration of PBMCs. The prophylactic administration of MB led to an increase of transendothelial migration of PBMCs but not Jurkat cells. Furthermore, HuMEC-1 secretion of cGMP correlated with iNOS expression after MB administration but not with eNOS expression. Expression of these molecules was reduced after MB administration at protein level. This study clearly reveals that endothelial response to MB is dose- and especially time-dependent. MB shows different effects on circulating blood cell-subtypes, and modifies the release patterns of eNOS, iNOS, and cGMP. The transendothelial migration is modulated after treatment with MB. Furthermore, MB provokes apoptosis of endothelial cells in a dose/time-dependent manner.
Conflict of interest statement
Figures
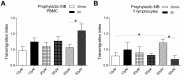
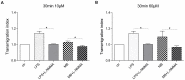
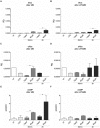
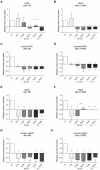
Similar articles
-
Methylene blue modulates adhesion molecule expression on microvascular endothelial cells.Inflamm Res. 2014 Aug;63(8):649-56. doi: 10.1007/s00011-014-0737-1. Epub 2014 May 3. Inflamm Res. 2014. PMID: 24794391
-
Treatment of endothelial cell with flavonoids modulates transendothelial leukocyte migration.Phlebology. 2015 Jul;30(6):405-11. doi: 10.1177/0268355514531951. Epub 2014 May 2. Phlebology. 2015. PMID: 24793119
-
Dose/time-dependent modulation of the endothelial function through induction agents: non-depleting versus depleting agents.Transplant Proc. 2014 Nov;46(9):2953-6. doi: 10.1016/j.transproceed.2014.06.055. Transplant Proc. 2014. PMID: 25420800
-
Reverse transendothelial cell migration in inflammation: to help or to hinder?Cell Mol Life Sci. 2017 May;74(10):1871-1881. doi: 10.1007/s00018-016-2444-2. Epub 2016 Dec 26. Cell Mol Life Sci. 2017. PMID: 28025672 Free PMC article. Review.
-
The regulation of transendothelial migration: new knowledge and new questions.Cardiovasc Res. 2015 Aug 1;107(3):310-20. doi: 10.1093/cvr/cvv145. Epub 2015 May 17. Cardiovasc Res. 2015. PMID: 25987544 Free PMC article. Review.
Cited by
-
Assessment of effects of methylene blue on intestinal ischemia and reperfusion in a rabbit model: hemodynamic, histological and immunohistochemical study.BMC Vet Res. 2020 Feb 12;16(1):54. doi: 10.1186/s12917-020-02279-6. BMC Vet Res. 2020. PMID: 32050965 Free PMC article.
-
Methylene Blue for Vasoplegic Syndrome After Cardiac Operation: Early Administration Improves Survival.Ann Thorac Surg. 2017 Jul;104(1):36-41. doi: 10.1016/j.athoracsur.2017.02.057. Epub 2017 May 24. Ann Thorac Surg. 2017. PMID: 28551045 Free PMC article.
-
Methylene blue modulates adhesion molecule expression on microvascular endothelial cells.Inflamm Res. 2014 Aug;63(8):649-56. doi: 10.1007/s00011-014-0737-1. Epub 2014 May 3. Inflamm Res. 2014. PMID: 24794391
-
Effect of Rewarming on Leukocyte-Endothelial Interaction After Deep Hypothermic Preservation.Ann Transplant. 2020 Feb 21;25:e919540. doi: 10.12659/AOT.919540. Ann Transplant. 2020. PMID: 32080161 Free PMC article.
-
Methylene blue: a controversial diagnostic acid and medication?Toxicol Res (Camb). 2022 Aug 30;11(5):711-717. doi: 10.1093/toxres/tfac050. eCollection 2022 Oct. Toxicol Res (Camb). 2022. PMID: 36337249 Free PMC article. Review.
References
-
- Levin MA, Lin HM, Castillo JG, Adams DH, Reich DL, et al. (2009) Early on-cardiopulmonary bypass hypotension and other factors associated with vasoplegic syndrome. Circulation 120: 1664–1671. - PubMed
-
- Levin RL, Degrange MA, Bruno GF, Del Mazo CD, Taborda DJ, et al. (2004) Methylene blue reduces mortality and morbidity in vasoplegic patients after cardiac surgery. Ann Thorac Surg 77: 496–499. - PubMed
-
- Ozal E, Kuralay E, Yildirim V, Kilic S, Bolcal C, et al. (2005) Preoperative methylene blue administration in patients at high risk for vasoplegic syndrome during cardiac surgery. Ann Thorac Surg 79: 1615–1619. - PubMed
-
- Shanmugam G (2005) Vasoplegic syndrome—the role of methylene blue. Eur J Cardiothorac Surg 28: 705–710. - PubMed
-
- Lenglet S, Mach F, Montecucco F (2011) Methylene blue: potential use of an antique molecule in vasoplegic syndrome during cardiac surgery. Expert Rev Cardiovasc Ther 9: 1519–1525. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

