Bone marrow stromal cells derived MCP-1 reverses the inhibitory effects of multiple myeloma cells on osteoclastogenesis by upregulating the RANK expression
- PMID: 24340030
- PMCID: PMC3858321
- DOI: 10.1371/journal.pone.0082453
Bone marrow stromal cells derived MCP-1 reverses the inhibitory effects of multiple myeloma cells on osteoclastogenesis by upregulating the RANK expression
Abstract
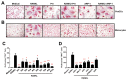
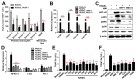
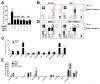
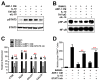
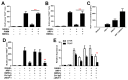
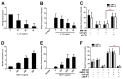
Multiple myeloma (MM) cells are responsible for aberrant osteoclast (OC) activation. However, when cocultured monocytes, but not OC precursors, with MM cells, we made a novel observation that MM cells inhibited receptor activator of nuclear factor κB ligand (RANKL)-induced increase of OC differentiation, OC gene expression, signaling pathways and bone resorption activity. Our results showed that MM cells produced multiple inhibitory cytokines of osteoclastogenesis, such as IL-10, which activated STAT3 signaling and induce OC inhibition. However, cocultures of bone marrow stromal cells (BMSCs) reversed MM-induced OC inhibition. We found that MM cells increased production of MCP-1 from BMSCs and BMSC-derived MCP-1 enhanced OC formation. Mechanistic studies showed that IL-10 downregulated RANK expression in monocytes and thus, inhibited RANKL-induced OC formation. In contrast, MCP-1 upregulated RANK expression and thus, enhanced OC formation. Overall, our studies for the first time demonstrated that MM cell have inhibitory effects on osteoclastogenesis by producing inhibitory cytokines. Our results further indicate that activation of osteoclastogenesis in bone marrow requests the crosstalk of MM cells, BMSCs and their produced cytokines. Thus, our studies provide evidences that targeting bone marrow microenvironmental cells and/or cytokines may be a new approach to treating MM bone destruction.
Conflict of interest statement
Figures

Similar articles
-
Expression of XBP1s in bone marrow stromal cells is critical for myeloma cell growth and osteoclast formation.Blood. 2012 May 3;119(18):4205-14. doi: 10.1182/blood-2011-05-353300. Epub 2012 Mar 16. Blood. 2012. PMID: 22427205 Free PMC article.
-
The RANK/RANK ligand system is involved in interleukin-6 and interleukin-11 up-regulation by human myeloma cells in the bone marrow microenvironment.Haematologica. 2004 Sep;89(9):1118-23. Haematologica. 2004. PMID: 15377473
-
Human myeloma cells stimulate the receptor activator of nuclear factor-kappa B ligand (RANKL) in T lymphocytes: a potential role in multiple myeloma bone disease.Blood. 2002 Dec 15;100(13):4615-21. doi: 10.1182/blood-2002-04-1121. Epub 2002 Aug 8. Blood. 2002. PMID: 12393684
-
RANK-Fc: a therapeutic antagonist for RANK-L in myeloma.Cancer. 2003 Feb 1;97(3 Suppl):802-12. doi: 10.1002/cncr.11134. Cancer. 2003. PMID: 12548579 Review.
-
New insight in the mechanism of osteoclast activation and formation in multiple myeloma: focus on the receptor activator of NF-kappaB ligand (RANKL).Exp Hematol. 2004 Aug;32(8):685-91. doi: 10.1016/j.exphem.2004.03.015. Exp Hematol. 2004. PMID: 15308315 Review.
Cited by
-
Plasma Levels of Monocyte Chemotactic Protein-1 Are Associated with Clinical Features and Angiogenesis in Patients with Multiple Myeloma.Biomed Res Int. 2016;2016:7870590. doi: 10.1155/2016/7870590. Epub 2016 Jan 27. Biomed Res Int. 2016. PMID: 26925413 Free PMC article.
-
Prospects and Advances in Adoptive Natural Killer Cell Therapy for Unmet Therapeutic Needs in Pediatric Bone Sarcomas.Int J Mol Sci. 2023 May 5;24(9):8324. doi: 10.3390/ijms24098324. Int J Mol Sci. 2023. PMID: 37176035 Free PMC article. Review.
-
Predicting chemotherapy toxicity in multiple myeloma: the prognostic value of pre-treatment serum cytokine levels of interleukin-6, interleukin-8, monocyte chemoattractant protein-1, and vascular endothelial growth factor.Front Immunol. 2024 May 23;15:1377546. doi: 10.3389/fimmu.2024.1377546. eCollection 2024. Front Immunol. 2024. PMID: 38846938 Free PMC article.
-
Study of the incidence of osteoporosis in patients with Sjögren's syndrome (pSS) and investigation of activation of the RANKL /RANK and osteoprotegerin (OPG) system.Mediterr J Rheumatol. 2018 Dec 18;29(4):224-227. doi: 10.31138/mjr.29.4.224. eCollection 2018 Dec. Mediterr J Rheumatol. 2018. PMID: 32185332 Free PMC article. No abstract available.
-
The Roles of ROS Generation in RANKL-Induced Osteoclastogenesis: Suppressive Effects of Febuxostat.Cancers (Basel). 2020 Apr 9;12(4):929. doi: 10.3390/cancers12040929. Cancers (Basel). 2020. PMID: 32283857 Free PMC article.
References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous

