Applying physics-based scoring to calculate free energies of binding for single amino acid mutations in protein-protein complexes
- PMID: 24340062
- PMCID: PMC3858304
- DOI: 10.1371/journal.pone.0082849
Applying physics-based scoring to calculate free energies of binding for single amino acid mutations in protein-protein complexes
Abstract
Predicting changes in protein binding affinity due to single amino acid mutations helps us better understand the driving forces underlying protein-protein interactions and design improved biotherapeutics. Here, we use the MM-GBSA approach with the OPLS2005 force field and the VSGB2.0 solvent model to calculate differences in binding free energy between wild type and mutant proteins. Crucially, we made no changes to the scoring model as part of this work on protein-protein binding affinity--the energy model has been developed for structure prediction and has previously been validated only for calculating the energetics of small molecule binding. Here, we compare predictions to experimental data for a set of 418 single residue mutations in 21 targets and find that the MM-GBSA model, on average, performs well at scoring these single protein residue mutations. Correlation between the predicted and experimental change in binding affinity is statistically significant and the model performs well at picking "hotspots," or mutations that change binding affinity by more than 1 kcal/mol. The promising performance of this physics-based method with no tuned parameters for predicting binding energies suggests that it can be transferred to other protein engineering problems.
Conflict of interest statement
Figures


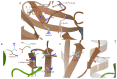
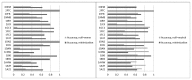

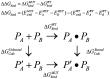
References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources

