SACE_5599, a putative regulatory protein, is involved in morphological differentiation and erythromycin production in Saccharopolyspora erythraea
- PMID: 24341557
- PMCID: PMC3878487
- DOI: 10.1186/1475-2859-12-126
SACE_5599, a putative regulatory protein, is involved in morphological differentiation and erythromycin production in Saccharopolyspora erythraea
Abstract
Background: Erythromycin is a medically important antibiotic, biosynthesized by the actinomycete Saccharopolyspora erythraea. Genes encoding erythromycin biosynthesis are organized in a gene cluster, spanning over 60 kbp of DNA. Most often, gene clusters encoding biosynthesis of secondary metabolites contain regulatory genes. In contrast, the erythromycin gene cluster does not contain regulatory genes and regulation of its biosynthesis has therefore remained poorly understood, which has for a long time limited genetic engineering approaches for erythromycin yield improvement.
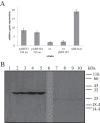
Results: We used a comparative proteomic approach to screen for potential regulatory proteins involved in erythromycin biosynthesis. We have identified a putative regulatory protein SACE_5599 which shows significantly higher levels of expression in an erythromycin high-producing strain, compared to the wild type S. erythraea strain. SACE_5599 is a member of an uncharacterized family of putative regulatory genes, located in several actinomycete biosynthetic gene clusters. Importantly, increased expression of SACE_5599 was observed in the complex fermentation medium and at controlled bioprocess conditions, simulating a high-yield industrial fermentation process in the bioreactor. Inactivation of SACE_5599 in the high-producing strain significantly reduced erythromycin yield, in addition to drastically decreasing sporulation intensity of the SACE_5599-inactivated strains when cultivated on ABSM4 agar medium. In contrast, constitutive overexpression of SACE_5599 in the wild type NRRL23338 strain resulted in an increase of erythromycin yield by 32%. Similar yield increase was also observed when we overexpressed the bldD gene, a previously identified regulator of erythromycin biosynthesis, thereby for the first time revealing its potential for improving erythromycin biosynthesis.
Conclusions: SACE_5599 is the second putative regulatory gene to be identified in S. erythraea which has positive influence on erythromycin yield. Like bldD, SACE_5599 is involved in morphological development of S. erythraea, suggesting a very close relationship between secondary metabolite biosynthesis and morphological differentiation in this organism. While the mode of action of SACE_5599 remains to be elucidated, the manipulation of this gene clearly shows potential for improvement of erythromycin production in S. erythraea in industrial setting. We have also demonstrated the applicability of the comparative proteomics approach for identifying new regulatory elements involved in biosynthesis of secondary metabolites in industrial conditions.
Figures
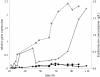
 ) and erythromycin concentration (
) and erythromycin concentration ( ) in the ABE1441 strain. Black lines represent relative expression of SACE_5599 (
) in the ABE1441 strain. Black lines represent relative expression of SACE_5599 ( ) and erythromycin concentration (
) and erythromycin concentration ( ) in the WT strain.
) in the WT strain.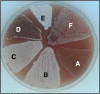

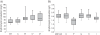
References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources

