Oral cyclosporine treatment in dogs: a review of the literature
- PMID: 24341787
- PMCID: PMC4895546
- DOI: 10.1111/jvim.12265
Oral cyclosporine treatment in dogs: a review of the literature
Abstract
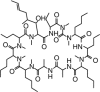
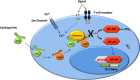
Cyclosporine is an immunomodulatory drug used to treat an increasing spectrum of diseases in dogs. Cyclosporine is a calcineurin inhibitor, ultimately exerting its inhibitory effects on T-lymphocytes by decreasing production of cytokines, such as interleukin-2. Although, in the United States, oral cyclosporine is approved in dogs only for treatment of atopic dermatitis, there are many other indications for its use. Cyclosporine is available in 2 oral formulations: the original oil-based formulation and the more commonly used ultramicronized emulsion that facilitates oral absorption. Ultramicronized cyclosporine is available as an approved animal product, and human proprietary and generic preparations are also available. Bioavailability of the different formulations in dogs is likely to vary among the preparations. Cyclosporine is associated with a large number of drug interactions that can also influence blood cyclosporine concentrations. Therapeutic drug monitoring (TDM) can be used to assist in attaining consistent plasma cyclosporine concentrations despite the effects of varying bioavailability and drug interactions. TDM can facilitate therapeutic success by guiding dose adjustments on an individualized basis, and is recommended in cases that do not respond to initial oral dosing, or during treatment of severe, life-threatening diseases for which a trial-and-error approach to dose adjustment is too risky. Pharmacodynamic assays that evaluate individual patient immune responses to cyclosporine can be used to augment information provided by TDM.
Keywords: Cyclosporine; Pharmacodynamics; Pharmacokinetics; Therapeutic drug monitoring.
Copyright © 2013 by the American College of Veterinary Internal Medicine.
Figures
Similar articles
-
Cyclosporin A pharmacokinetics and efficacy in the treatment of atopic dermatitis in dogs.J Vet Pharmacol Ther. 2004 Aug;27(4):231-8. doi: 10.1111/j.1365-2885.2004.00587.x. J Vet Pharmacol Ther. 2004. PMID: 15305852 Clinical Trial.
-
Cyclosporine and tacrolimus.Semin Vet Med Surg Small Anim. 1997 Aug;12(3):161-6. doi: 10.1016/s1096-2867(97)80028-x. Semin Vet Med Surg Small Anim. 1997. PMID: 9283240 Review.
-
Concurrent administration of water-soluble vitamin E can increase the oral bioavailability of cyclosporine a in healthy dogs.Vet Ther. 2002 Winter;3(4):465-73. Vet Ther. 2002. PMID: 12584684 Clinical Trial.
-
Improving the oral absorption of cyclosporine in malabsorbers: a review of the Neoral Compassionate Need Program.Transplant Proc. 1998 Aug;30(5):1691-3. doi: 10.1016/s0041-1345(98)00395-9. Transplant Proc. 1998. PMID: 9723246 Clinical Trial. No abstract available.
-
More economical use of cyclosporine through combination drug therapy.J Am Anim Hosp Assoc. 2002 May-Jun;38(3):205-8. doi: 10.5326/0380205. J Am Anim Hosp Assoc. 2002. PMID: 12022403 Review. No abstract available.
Cited by
-
A case of pulmonary toxoplasmosis resembling multiple lung metastases of nasal lymphoma in a cat receiving chemotherapy.J Vet Med Sci. 2018 Dec 26;80(12):1881-1886. doi: 10.1292/jvms.18-0340. Epub 2018 Nov 8. J Vet Med Sci. 2018. PMID: 30404954 Free PMC article.
-
Hypertensive nonobstructive hydrocephalus as main magnetic resonance imaging feature in a dog with disseminated choroid plexus carcinomatosis.J Vet Intern Med. 2023 Jul-Aug;37(4):1493-1500. doi: 10.1111/jvim.16737. Epub 2023 May 24. J Vet Intern Med. 2023. PMID: 37224288 Free PMC article.
-
Recovery of T-cell function in healthy dogs following cessation of oral cyclosporine administration.Vet Med Sci. 2020 Aug;6(3):277-282. doi: 10.1002/vms3.230. Epub 2020 Jan 8. Vet Med Sci. 2020. PMID: 31914237 Free PMC article.
-
Immunosuppression for in vivo research: state-of-the-art protocols and experimental approaches.Cell Mol Immunol. 2017 Feb;14(2):146-179. doi: 10.1038/cmi.2016.39. Epub 2016 Oct 10. Cell Mol Immunol. 2017. PMID: 27721455 Free PMC article. Review.
-
A Retrospective Evaluation of the Steroid-Sparing Effect of Oral Modified Ciclosporin for Treatment of Canine Pemphigus Foliaceus.Vet Sci. 2022 Mar 23;9(4):153. doi: 10.3390/vetsci9040153. Vet Sci. 2022. PMID: 35448651 Free PMC article.
References
-
- Kahan BD. Therapeutic drug monitoring of cyclosporine: 20 years of progress. Transplant Proc 2004;36:378S–391S. - PubMed
-
- Calne R. Cyclosporine as a milestone in immunosuppression. Transplant Proc 2004;36:13S–15S. - PubMed
-
- Graeb C, Arbogast H, Guba M, et al. Cyclosporine: 20 years of experience at the University of Munich. Transplant Proc 2004;36:125S–129S. - PubMed
-
- Citterio F. Evolution of the therapeutic drug monitoring of cyclosporine. Transplant Proc 2004;36:420S–425S. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical