Lipids and topological rules governing membrane protein assembly
- PMID: 24341994
- PMCID: PMC4057987
- DOI: 10.1016/j.bbamcr.2013.12.007
Lipids and topological rules governing membrane protein assembly
Abstract
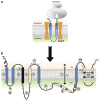
Membrane protein folding and topogenesis are tuned to a given lipid profile since lipids and proteins have co-evolved to follow a set of interdependent rules governing final protein topological organization. Transmembrane domain (TMD) topology is determined via a dynamic process in which topogenic signals in the nascent protein are recognized and interpreted initially by the translocon followed by a given lipid profile in accordance with the Positive Inside Rule. The net zero charged phospholipid phosphatidylethanolamine and other neutral lipids dampen the translocation potential of negatively charged residues in favor of the cytoplasmic retention potential of positively charged residues (Charge Balance Rule). This explains why positively charged residues are more potent topological signals than negatively charged residues. Dynamic changes in orientation of TMDs during or after membrane insertion are attributed to non-sequential cooperative and collective lipid-protein charge interactions as well as long-term interactions within a protein. The proportion of dual topological conformers of a membrane protein varies in a dose responsive manner with changes in the membrane lipid composition not only in vivo but also in vitro and therefore is determined by the membrane lipid composition. Switching between two opposite TMD topologies can occur in either direction in vivo and also in liposomes (designated as fliposomes) independent of any other cellular factors. Such lipid-dependent post-insertional reversibility of TMD orientation indicates a thermodynamically driven process that can occur at any time and in any cell membrane driven by changes in the lipid composition. This dynamic view of protein topological organization influenced by the lipid environment reveals previously unrecognized possibilities for cellular regulation and understanding of disease states resulting from mis-folded proteins. This article is part of a Special Issue entitled: Protein trafficking and secretion in bacteria. Guest Editors: Anastassios Economou and Ross Dalbey.
Keywords: Charge Balance Rule; Dual topology; Membrane protein topology; Phosphatidylethanolamine; Positive Inside Rule; Topogenesis.
Copyright © 2013 Elsevier B.V. All rights reserved.
Figures

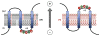

References
-
- Enquist K, Fransson M, Boekel C, Bengtsson I, Geiger K, Lang L, Pettersson A, Johansson S, von Heijne G, Nilsson I. Membrane-integration characteristics of two ABC transporters, CFTR and P-glycoprotein. J Mol Biol. 2009;387:1153–1164. - PubMed
-
- Lee E, Manoil C. Mutations eliminating the protein export function of a membrane-spanning sequence. J Biol Chem. 1994;269:28822–28828. - PubMed
-
- Hessa T, Kim H, Bihlmaier K, Lundin C, Boekel J, Andersson H, Nilsson I, White SH, von Heijne G. Recognition of transmembrane helices by the endoplasmic reticulum translocon. Nature. 2005;433:377–381. - PubMed
-
- Mackenzie KR. Folding and stability of alpha-helical integral membrane proteins. Chem Rev. 2006;106:1931–1977. - PubMed
-
- White SH, von Heijne G. Do protein–lipid interactions determine the recognition of transmembrane helices at the ER translocon? Biochem Soc Trans. 2005;33:1012–1015. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

