A bioimage informatics based reconstruction of breast tumor microvasculature with computational blood flow predictions
- PMID: 24342178
- PMCID: PMC3977934
- DOI: 10.1016/j.mvr.2013.12.003
A bioimage informatics based reconstruction of breast tumor microvasculature with computational blood flow predictions
Abstract
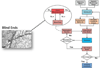
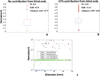
Induction of tumor angiogenesis is among the hallmarks of cancer and a driver of metastatic cascade initiation. Recent advances in high-resolution imaging enable highly detailed three-dimensional geometrical representation of the whole-tumor microvascular architecture. This enormous increase in complexity of image-based data necessitates the application of informatics methods for the analysis, mining and reconstruction of these spatial graph data structures. We present a novel methodology that combines ex-vivo high-resolution micro-computed tomography imaging data with a bioimage informatics algorithm to track and reconstruct the whole-tumor vasculature of a human breast cancer model. The reconstructed tumor vascular network is used as an input of a computational model that estimates blood flow in each segment of the tumor microvascular network. This formulation involves a well-established biophysical model and an optimization algorithm that ensures mass balance and detailed monitoring of all the vessels that feed and drain blood from the tumor microvascular network. Perfusion maps for the whole-tumor microvascular network are computed. Morphological and hemodynamic indices from different regions are compared to infer their role in overall tumor perfusion.
Keywords: Adjacency matrix; BFS; D; DFS; G; IQR; L; L(D); MPL; MTT; Minimum heap; N; P; Priority queue; Q; R; a symmetric matrix consisting of ones and zeros indicating whether or not 2 nodes are connected with a segment; breadth-first search traversal algorithm (systematic visiting of nodes and segments of the graph); data structure where each element is served based on a predefined priority; depth-first search traversal algorithm (systematic visiting of nodes and segments of the graph); flow rate; flow-weighted mean path length; hydraulic conductance; interquartile range (measure of data dispersion); m(tissue); mass of tumor tissue; maximum extravascular diffusion distance of oxygen; pressure; segment diameter; segment length; total mean transit time; total number of segments; tree-based data structure where the parent nodes have always an identity (key) smaller than their children nodes; vascular length density.
Copyright © 2013 Elsevier Inc. All rights reserved.
Figures






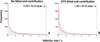



References
-
- Acker JC, et al. Blood perfusion measurements in human tumours: evaluation of laser Doppler methods. Int J Hyperthermia. 1990;6:287–304. - PubMed
-
- Beaney RP, et al. Positron emission tomography for in-vivo measurement of regional blood flow, oxygen utilisation, and blood volume in patients with breast carcinoma. Lancet. 1984;1:131–134. - PubMed
-
- Benedict KF, et al. Hemodynamic systems analysis of capillary network remodeling during the progression of type 2 diabetes. Microcirculation. 2011;18:63–73. - PubMed
-
- Bentley MD, et al. The use of microcomputed tomography to study microvasculature in small rodents. Am J Physiol Regul Integr Comp Physiol. 2002;282:R1267–R1279. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

