Randomized controlled trial of two dosing schedules for human papillomavirus vaccination among college age males
- PMID: 24342252
- PMCID: PMC4048919
- DOI: 10.1016/j.vaccine.2013.11.098
Randomized controlled trial of two dosing schedules for human papillomavirus vaccination among college age males
Abstract
Background: Quadrivalent human papillomavirus (HPV) vaccine, for protection against sexually transmitted HPV infection, is licensed for females and males 9-26 years on a 3-dose schedule (0, 2, and 6 months; Standard schedule). Vaccine uptake has been low and catch-up vaccination of older adolescents using an alternate dosing schedule may increase coverage. This study tested the non-inferiority of the immunogenicity of an alternate dosing schedule (0, 2, 12 months) among college age males.
Methods: 220 18-25 year old males were randomly assigned to Standard or Alternate schedules. Blood samples were drawn immediately before Dose 1 and 2-6 weeks after Dose 3 and analyzed for antibody titers using a Luminex immunoassay. A value <1.5 for the upper 95% confidence interval (CI) bound of the Standard to Alternate schedule geometric mean titer (GMT) ratio was deemed non-inferior.
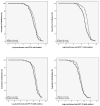
Results: Participants averaged 21.3 years old; 19.1% were non-white; completion rate was 93%. The anti-HPV titers for the Alternate schedule group were non-inferior to those of Standard schedule group for all four HPV vaccine virus types. Our results also demonstrated superiority of the Alternate schedule group for all four HPV vaccine virus types.
Conclusion: A delayed third dose at 12 months is immunologically non-inferior and superior for four HPV virus types. Using an alternate dosing schedule offers more flexibility to receive the 3-dose HPV vaccine and may result in higher vaccination rates among college-age males.
Trial registration: ClinicalTrials.gov NCT01184079.
Keywords: HPV vaccine; Human papillomavirus; Immunization; Non-inferiority.
Copyright © 2013 Elsevier Ltd. All rights reserved.
Conflict of interest statement
The authors have no other conflicts to report regarding HPV vaccine.
Figures

References
-
- US Food and Drug Administration. Vaccines, Blood & Biologics - Gardasil. http://www.fda.gov/BiologicsBloodVaccines/Vaccines/ApprovedProducts/UCM0... [Cited 2013, March 13]
-
- US Department of Health and Human Services. Healthy People 2020. http://www.healthypeople.gov/2020/topicsobjectives2020/objectiveslist.as... [Cited 2013, May 30]
-
- Centers for Disease Control and Prevention. National and state vaccination coverage among adolescents aged 13 through 17 years--United States. Morbidity and Mortality Weekly Report (MMWR) 2011;60:1117–23. - PubMed
-
- Kessels SJ, Marshall HS, Watson M, Braunack-Mayer AJ, Reuzel R, Tooher RL. Factors associated with HPV vaccine uptake in teenage girls: a systematic review. Vaccine. 2012;30:3546–56. - PubMed
Publication types
MeSH terms
Substances
Associated data
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

