Antibiotic activity against naive and induced Streptococcus pneumoniae biofilms in an in vitro pharmacodynamic model
- PMID: 24342635
- PMCID: PMC3957900
- DOI: 10.1128/AAC.01858-13
Antibiotic activity against naive and induced Streptococcus pneumoniae biofilms in an in vitro pharmacodynamic model
Abstract
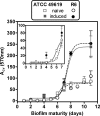
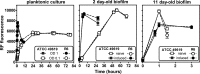
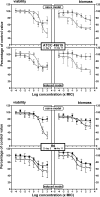
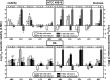
Biofilms play a role in the pathogenicity of pneumococcal infections. A pharmacodynamic in vitro model of biofilm was developed that allows characterization of the activity of antibiotics against viability and biomass by using in parallel capsulated (ATCC 49619) and noncapsulated (R6) reference strains. Naive biofilms were obtained by incubating fresh planktonic cultures for 2 to 11 days in 96-well polystyrene plates. Induced biofilms were obtained using planktonic bacteria collected from the supernatant of 6-day-old naive biofilms. Biomass production was more rapid and intense in the induced model, but the levels were similar for both strains. Full concentration responses fitting sigmoidal regressions allowed calculation of maximal efficacies and relative potencies of drugs. All antibiotics tested (amoxicillin, clarithromycin, solithromycin, levofloxacin, and moxifloxacin) were more effective against young naive biofilms than against old or induced biofilms, except macrolides/ketolides, which were as effective at reducing viability in 2-day-old naive biofilms and in 11-day-old induced biofilms of R6. Macrolides/ketolides, however, were less potent than fluoroquinolones against R6 (approximately 5- to 20-fold-higher concentrations needed to reduction viability of 20%). However, at concentrations obtainable in epithelial lining fluid, the viabilities of mature or induced biofilms were reduced 15 to 45% (amoxicillin), 17 to 44% (macrolides/ketolides), and 12 to 64% (fluoroquinolones), and biomasses were reduced 5 to 45% (amoxicillin), 5 to 60% (macrolides/ketolides), and 10 to 76% (fluoroquinolones), with solithromycin and moxifloxacin being the most effective and the most potent agents (due to lower MICs) in their respective classes. This study allowed the ranking of antibiotics with respect to their potential effectiveness in biofilm-related infections, underlining the need to search for still more effective options.
Figures



References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases

