Pre- and postnatal transplantation of fetal mesenchymal stem cells in osteogenesis imperfecta: a two-center experience
- PMID: 24342908
- PMCID: PMC3925052
- DOI: 10.5966/sctm.2013-0090
Pre- and postnatal transplantation of fetal mesenchymal stem cells in osteogenesis imperfecta: a two-center experience
Abstract
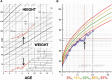
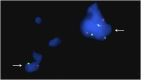
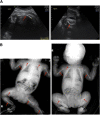
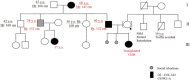
Osteogenesis imperfecta (OI) can be recognized prenatally with ultrasound. Transplantation of mesenchymal stem cells (MSCs) has the potential to ameliorate skeletal damage. We report the clinical course of two patients with OI who received prenatal human fetal MSC (hfMSC) transplantation and postnatal boosting with same-donor MSCs. We have previously reported on prenatal transplantation for OI type III. This patient was retransplanted with 2.8 × 10(6) same-donor MSCs per kilogram at 8 years of age, resulting in low-level engraftment in bone and improved linear growth, mobility, and fracture incidence. An infant with an identical mutation who did not receive MSC therapy succumbed at 5 months despite postnatal bisphosphonate therapy. A second fetus with OI type IV was also transplanted with 30 × 10(6) hfMSCs per kilogram at 31 weeks of gestation and did not suffer any new fractures for the remainder of the pregnancy or during infancy. The patient followed her normal growth velocity until 13 months of age, at which time longitudinal length plateaued. A postnatal infusion of 10 × 10(6) MSCs per kilogram from the same donor was performed at 19 months of age, resulting in resumption of her growth trajectory. Neither patient demonstrated alloreactivity toward the donor hfMSCs or manifested any evidence of toxicities after transplantation. Our findings suggest that prenatal transplantation of allogeneic hfMSCs in OI appears safe and is of likely clinical benefit and that retransplantation with same-donor cells is feasible. However, the limited experience to date means that it is not possible to be conclusive and that further studies are required.
Keywords: Cell therapy; In utero transplantation; Mesenchymal stem cells; Mesenchymal stromal cells; Osteogenesis imperfecta; Prenatal transplantation.
Figures

References
-
- Steiner RD, Pepin MG, Byers PH. Osteogenesis Imperfecta. In: Pagon RA, Bird TD, Dolan CR et al., eds. GeneReviews. Seattle, WA: University of Washington, Seattle, 1993.
-
- Glorieux FH, Bishop NJ, Plotkin H, et al. Cyclic administration of pamidronate in children with severe osteogenesis imperfecta. N Engl J Med. 1998;339:947–952. - PubMed
-
- Bishop N. Characterising and treating osteogenesis imperfecta. Early Hum Dev. 2010;86:743–746. - PubMed
-
- Pittenger MF, Mackay AM, Beck SC, et al. Multilineage potential of adult human mesenchymal stem cells. Science. 1999;284:143–147. - PubMed
Publication types
MeSH terms
Supplementary concepts
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

