Sorbin and SH3 domain-containing protein 2 is released from infarcted heart in the very early phase: proteomic analysis of cardiac tissues from patients
- PMID: 24342996
- PMCID: PMC3886759
- DOI: 10.1161/JAHA.113.000565
Sorbin and SH3 domain-containing protein 2 is released from infarcted heart in the very early phase: proteomic analysis of cardiac tissues from patients
Abstract
Background: Few proteomic studies have examined human cardiac tissue following acute lethal infarction. Here, we applied a novel proteomic approach to formalin-fixed, paraffin-embedded human tissue and aimed to reveal the molecular changes in the very early phase of acute myocardial infarction.
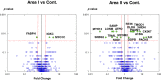
Methods and results: Heart tissue samples were collected from 5 patients who died within 7 hours of myocardial infarction and from 5 age- and sex-matched control cases. Infarcted and control myocardia were histopathologically diagnosed and captured using laser microdissection. Proteins were extracted using an originally established method and analyzed using liquid chromatography-tandem mass spectrometry. The label-free quantification demonstrated that the levels of 21 proteins differed significantly between patients and controls. In addition to known biomarkers, the sarcoplasmic protein sorbin and SH3 domain-containing protein 2 (SORBS2) was greatly reduced in infarcted myocardia. Immunohistochemical analysis of cardiac tissues confirmed the decrease, and Western blot analysis showed a significant increase in serum sorbin and SH3 domain-containing protein 2 in acute myocardial infarction patients (n=10) compared with control cases (n=11).
Conclusions: Our advanced comprehensive analysis using patient tissues and serums indicated that sarcoplasmic sorbin and SH3 domain-containing protein 2 is released from damaged cardiac tissue into the bloodstream upon lethal acute myocardial infarction. The proteomic strategy presented here is based on precise microscopic findings and is quite useful for candidate biomarker discovery using human tissue samples stored in depositories.
Keywords: SORBS2; myocardial infarction; proteomics; tissue.
Figures




References
-
- Theilmeier G, Schmidt C, Herrmann J, Keul P, Schafers M, Herrgott I, Mersmann J, Larmann J, Hermann S, Stypmann J, Schober O, Hildebrand R, Schulz R, Heusch G, Haude M, von Wnuck Lipinski K, Herzog C, Schmitz M, Erbel R, Chun J, Levkau B. High‐density lipoproteins and their constituent, sphingosine‐1‐phosphate, directly protect the heart against ischemia/reperfusion injury in vivo via the S1P3 lysophospholipid receptor. Circulation. 2006; 114:1403-1409 - PubMed
-
- Barallobre‐Barreiro J, Didangelos A, Schoendube FA, Drozdov I, Yin X, Fernandez‐Caggiano M, Willeit P, Puntmann VO, Aldama‐Lopez G, Shah AM, Domenech N, Mayr M. Proteomics analysis of cardiac extracellular matrix remodeling in a porcine model of ischemia/reperfusion injury. Circulation. 2012; 125:789-802 - PubMed
-
- Meune C, Twerenbold R, Drexler B, Balmelli C, Wolf C, Haaf P, Reichlin T, Irfan A, Reiter M, Zellweger C, Meissner J, Stelzig C, Freese M, Capodarve I, Mueller C. Midregional pro‐A‐type natriuretic peptide for diagnosis and prognosis in patients with suspected acute myocardial infarction. Am J Cardiol. 2012; 109:1117-1123 - PubMed
-
- Tiwari RP, Jain A, Khan Z, Kohli V, Bharmal RN, Kartikeyan S, Bisen PS. Cardiac troponins I and T: molecular markers for early diagnosis, prognosis, and accurate triaging of patients with acute myocardial infarction. Mol Diagn Ther. 2012; 16:371-381 - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

