Circulating angiotensin II gains access to the hypothalamus and brain stem during hypertension via breakdown of the blood-brain barrier
- PMID: 24343120
- PMCID: PMC4080808
- DOI: 10.1161/HYPERTENSIONAHA.113.01743
Circulating angiotensin II gains access to the hypothalamus and brain stem during hypertension via breakdown of the blood-brain barrier
Abstract
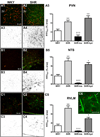
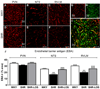
Angiotensin II-mediated vascular brain inflammation emerged as a novel pathophysiological mechanism in neurogenic hypertension. However, the precise underlying mechanisms and functional consequences in relation to blood-brain barrier (BBB) integrity and central angiotensin II actions mediating neurohumoral activation in hypertension are poorly understood. Here, we aimed to determine whether BBB permeability within critical hypothalamic and brain stem regions involved in neurohumoral regulation was altered during hypertension. Using digital imaging quantification after intravascularly injected fluorescent dyes and immunohistochemistry, we found increased BBB permeability, along with altered key BBB protein constituents, in spontaneously hypertensive rats within the hypothalamic paraventricular nucleus, the nucleus of the solitary tract, and the rostral ventrolateral medulla, all critical brain regions known to contribute to neurohumoral activation during hypertension. BBB disruption, including increased permeability and downregulation of constituent proteins, was prevented in spontaneously hypertensive rats treated with the AT1 receptor antagonist losartan, but not with hydralazine, a direct vasodilator. Importantly, we found circulating angiotensin II to extravasate into these brain regions, colocalizing with neurons and microglial cells. Taken together, our studies reveal a novel angiotensin II-mediated feed-forward mechanism during hypertension, by which circulating angiotensin II evokes increased BBB permeability, facilitating in turn its access to critical brain regions known to participate in blood pressure regulation.
Keywords: angiotensin II; blood–brain barrier; brain stem; hypertension; hypothalamus; receptor, angiotensin, type 1.
Conflict of interest statement
None.
Figures



References
-
- Guyenet PG. The sympathetic control of blood pressure. Nat Rev Neurosci. 2006;7:335–346. - PubMed
-
- McKinley MJ, McAllen RM, Pennington GL, Smardencas A, Weisinger RS, Oldfield BJ. Physiological actions of angiotensin II mediated by at1 and at2 receptors in the brain. Clin Exp Pharmacol Physiol Suppl. 1996;3:S99–S104. - PubMed
-
- Zardetto-Smith AM, Thunhorst RL, Cicha MZ, Johnson AK. Afferent signaling and forebrain mechanisms in the behavioral control of extracellular fluid volume. Ann N Y Acad Sci. 1993;689:161–176. - PubMed
-
- Smith PM, Ferguson AV. Circulating signals as critical regulators of autonomic state--central roles for the subfornical organ. Am J Physiol Regul Integr Comp Physiol. 2010;299:R405–R415. - PubMed
-
- Ferguson AV, Bains JS. Actions of angiotensin in the subfornical organ and area postrema: Implications for long term control of autonomic output. Clin Exp Pharmacol Physiol. 1997;24:96–101. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials

