Low-dose intradermal infection with trypanosoma congolense leads to expansion of regulatory T cells and enhanced susceptibility to reinfection
- PMID: 24343657
- PMCID: PMC3957988
- DOI: 10.1128/IAI.01028-13
Low-dose intradermal infection with trypanosoma congolense leads to expansion of regulatory T cells and enhanced susceptibility to reinfection
Abstract
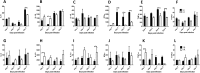
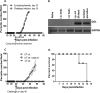
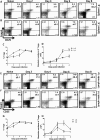
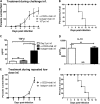
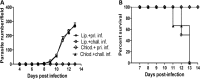
BALB/c mice are highly susceptible to experimental intraperitoneal Trypanosoma congolense infection. However, a recent report showed that these mice are relatively resistant to primary intradermal low-dose infection. Paradoxically, repeated low-dose intradermal infections predispose mice to enhanced susceptibility to an otherwise noninfectious dose challenge. Here, we explored the mechanisms responsible for this low-dose-induced susceptibility to subsequent low-dose challenge infection. We found that akin to intraperitoneal infection, low-dose intradermal infection led to production of interleukin-10 (IL-10), IL-6, IL-12, tumor necrosis factor alpha (TNF-α), transforming growth factor β (TGF-β), and gamma interferon (IFN-γ) by spleen and draining lymph node cells. Interestingly, despite the absence of parasitemia, low-dose intradermal infection led to expansion of CD4+ CD25+ Foxp3+ cells (T regulatory cells [Tregs]) in both the spleens and lymph nodes draining the infection site. Depletion of Tregs by anti-CD25 monoclonal antibody (MAb) treatment during primary infection or before challenge infection following repeated low-dose infection completely abolished the low-dose-induced enhanced susceptibility. In addition, Treg depletion was associated with dramatic reduction in serum levels of TGF-β and IL-10. Collectively, these findings show that low-dose intradermal infection leads to rapid expansion of Tregs, and these cells mediate enhanced susceptibility to subsequent infection.
Figures


References
-
- Kristjanson PM, Swallow BM, Rowlands GJ, Kruska RL, de Leeuw PN. 1999. Measuring the costs of African animal trypanosomosis: the potential benefits of control and returns to research. Agric. Syst. 59:79–98. 10.1016/S0308-521X(98)00086-9 - DOI
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials

