Rosetting Plasmodium falciparum-infected erythrocytes bind to human brain microvascular endothelial cells in vitro, demonstrating a dual adhesion phenotype mediated by distinct P. falciparum erythrocyte membrane protein 1 domains
- PMID: 24343658
- PMCID: PMC3958005
- DOI: 10.1128/IAI.01233-13
Rosetting Plasmodium falciparum-infected erythrocytes bind to human brain microvascular endothelial cells in vitro, demonstrating a dual adhesion phenotype mediated by distinct P. falciparum erythrocyte membrane protein 1 domains
Abstract
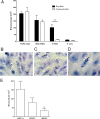
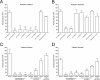
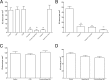
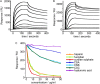
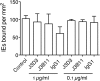
Adhesion interactions between Plasmodium falciparum-infected erythrocytes (IE) and human cells underlie the pathology of severe malaria. IE cytoadhere to microvascular endothelium or form rosettes with uninfected erythrocytes to survive in vivo by sequestering IE in the microvasculature and avoiding splenic clearance mechanisms. Both rosetting and cytoadherence are mediated by the parasite-derived IE surface protein family Plasmodium falciparum erythrocyte membrane protein 1 (PfEMP1). Rosetting and cytoadherence have been widely studied as separate entities; however, the ability of rosetting P. falciparum strains to cytoadhere has received little attention. Here, we show that IE of the IT/R29 strain expressing a rosette-mediating PfEMP1 variant (IT4var09) cytoadhere in vitro to a human brain microvascular endothelial cell line (HBEC-5i). Cytoadherence was inhibited by heparin and by treatment of HBEC-5i with heparinase III, suggesting that the endothelial receptors for IE binding are heparan sulfate proteoglycans. Antibodies to the N-terminal regions of the IT4var09 PfEMP1 variant (NTS-DBL1α and DBL2γ domains) specifically inhibited and reversed cytoadherence down to low concentrations (<10 μg/ml of total IgG). Surface plasmon resonance experiments showed that the NTS-DBLα and DBL2γ domains bind strongly to heparin, with half-maximal binding at a concentration of ∼0.5 μM in both cases. Therefore, cytoadherence of IT/R29 IE is distinct from rosetting, which is primarily mediated by NTS-DBL1α interactions with complement receptor 1. These data show that IT4var09-expressing parasites are capable of dual interactions with both endothelial cells and uninfected erythrocytes via distinct receptor-ligand interactions.
Figures

References
-
- Su X-Z, Heatwole VM, Wertheimer SP, Guinet F, Herrfeldt JA, Peterson DS, Ravetch JA, Wellems TE. 1995. A large and diverse family gene family (var) encodes 200–350 kD proteins implicated in the antigenic variation and cytoadherence of Plasmodium falciparum-infected erythrocytes. Cell 82:89–99. 10.1016/0092-8674(95)90055-1 - DOI - PubMed
-
- Smith JD, Chitnis CE, Craig AG, Roberts DJ, Hudson-Taylor DE, Peterson DS, Pinches R, Newbold CI, Miller LH. 1995. Switches in expression of Plasmodium falciparum var genes correlate with changes in antigenic and cytoadherent phenotypes of infected erythrocytes. Cell 82:101–110. 10.1016/0092-8674(95)90056-X - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials

