Hyaluronidase-sensitive nanoparticle templates for triggered release of HIV/AIDS microbicide in vitro
- PMID: 24343770
- PMCID: PMC3933588
- DOI: 10.1208/s12248-013-9546-7
Hyaluronidase-sensitive nanoparticle templates for triggered release of HIV/AIDS microbicide in vitro
Abstract
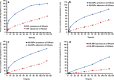
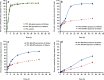
This study was designed to test the hypothesis that a triggered release of a topical microbicide (tenofovir) from hyaluronic acid nanoparticles (HA-NPs) can be achieved under the influence of hyaluronidase (HAase) enzyme. A fractional factorial experimental design was used to examine the factors [molar concentrations of adipic acid dihydrazide (X1) and 1-ethyl-3-[3-dimethylaminopropyl] carbodiimide hydrochloride (X2), volume of acetone (X3) and reaction time (X4)] influencing the responses, Y1; particle mean diameter: PMD (nanometers: nm), Y2; polydispersity index: PDI and Y3; zeta (ζ) potential: (millivolts). The amide bond formation between HA and ADH after cross-linking was confirmed by FT-IR and (13)C-NMR analyses. These NPs were also characterized for cytotoxicity on a human vaginal epithelial cell line and L. crispatus. When formulated with factors X1; 2.49 mM, X2; 9.96 mM, X3; 60 mL, X4; 6 h, HA-NPs exhibited a spherical shape with PMD, PDI, ζ potential, encapsulation efficiency, and drug loading of 70.6 ± 4.1 nm, 0.07 ± 0.02, -38.2 ± 2.8 mV, 51.8 ± 2.4% w/w and 26.1 ± 1.2% w/w, respectively, (n = 3). Unlike for HA based gel, HAase significantly triggered the drug release and HA degradation from the NPs after 24 h (~90% w/w and 65% w/w, respectively); whereas, in its absence, these values were ~39% w/w and 26% w/w, respectively. The NPs were non-cytotoxic to human vaginal VK2/E6E7, End1/E6E7 cells and Lactobacillus crispatus. These data highlight the potential of HAase-sensitive HA-NPs templates for the controlled and vaginal delivery of anti-HIV/AIDS microbicides.
Figures



References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases
Research Materials

