Agomelatine versus other antidepressive agents for major depression
- PMID: 24343836
- PMCID: PMC11289707
- DOI: 10.1002/14651858.CD008851.pub2
Agomelatine versus other antidepressive agents for major depression
Abstract
Background: Major depressive disorder (MDD), or depression, is a syndrome characterised by a number of behavioural, cognitive and emotional features. It is most commonly associated with a sad or depressed mood, a reduced capacity to feel pleasure, feelings of hopelessness, loss of energy, altered sleep patterns, weight fluctuations, difficulty in concentrating and suicidal ideation. There is a need for more effective and better tolerated antidepressants to combat this condition. Agomelatine was recently added to the list of available antidepressant drugs; it is a novel antidepressant that works on melatonergic (MT1 and MT2), 5-HT 2B and 5-HT2C receptors. Because the mechanism of action is claimed to be novel, it may provide a useful, alternative pharmacological strategy to existing antidepressant drugs.
Objectives: The objective of this review was 1) to determine the efficacy of agomelatine in alleviating acute symptoms of major depressive disorder in comparison with other antidepressants, 2) to review the acceptability of agomelatine in comparison with other antidepressant drugs, and, 3) to investigate the adverse effects of agomelatine, including the general prevalence of side effects in adults.
Search methods: We searched the Cochrane Collaboration's Depression, Anxiety and Neurosis Review Group's Specialised Register (CCDANCTR) to 31 July 2013. The CCDANCTR includes relevant randomised controlled trials from the following bibliographic databases: CENTRAL (the Cochrane Central Register of Controlled Trials) (all years), EMBASE (1974 onwards), MEDLINE (1950 onwards) and PsycINFO (1967 onwards). We checked reference lists of relevant studies together with reviews and regulatory agency reports. No restrictions on date, language or publication status were applied to the search. Servier Laboratories (developers of agomelatine) and other experts in the field were contacted for supplemental data.
Selection criteria: Randomised controlled trials allocating adult participants with major depression to agomelatine versus any other antidepressive agent.
Data collection and analysis: Two review authors independently extracted data and a double-entry procedure was employed. Information extracted included study characteristics, participant characteristics, intervention details and outcome measures in terms of efficacy, acceptability and tolerability.
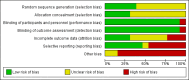
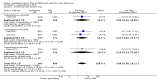
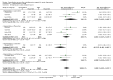
Main results: A total of 13 studies (4495 participants) were included in this review. Agomelatine was compared to selective serotonin reuptake inhibitors (SSRIs), namely paroxetine, fluoxetine, sertraline, escitalopram, and to the serotonin-norepinephrine reuptake inhibitor (SNRI), venlafaxine. Participants were followed up for six to 12 weeks. Agomelatine did not show any advantage or disadvantage over the other antidepressants for our primary outcome, response to treatment (risk ratio (RR) 1.01, 95% confidence interval (CI) 0.95 to 1.08, P value 0.75 compared to SSRIs, and RR 1.06; 95% CI 0.98 to 1.16, P value 0.16 compared to venlafaxine). Also, agomelatine showed no advantage or disadvantage over other antidepressants for remission (RR 0.83; 95% CI 0.68 to 1.01, P value 0.07 compared to SSRIs, and RR 1.08; 95% CI 0.94 to 1.24, P value 0.73 compared to venlafaxine). Overall, agomelatine appeared to be better tolerated than venlafaxine in terms of lower rates of drop outs (RR 0.40; 95% CI 0.24 to 0.67, P value 0.0005), and showed the same level of tolerability as SSRIs (RR 0.95; 95% CI 0.83 to 1.09, P value 0.44). Agomelatine induced a lower rate of dizziness than venlafaxine (RR 0.19, 95% CI 0.06 to 0.64, P value 0.007).With regard to the quality of the body of evidence, there was a moderate risk of bias for all outcomes, due to the number of included unpublished studies. There was some heterogeneity, particularly between published and unpublished studies. The included studies were conducted in inpatient and outpatient settings, thus limiting the generalisability of the results to primary care settings. With regard to precision, the efficacy outcomes were precise, but the tolerability outcomes were mostly imprecise. Publication bias was variable and depended on the outcome of the trial. Our review included unpublished studies, and we think that this reduced the impact of publication bias. The overall methodological quality of the studies was not very good. Almost all of the studies were sponsored by the pharmaceutical company that manufactures agomelatine (Servier), and some of these were unpublished. Attempts to contact the pharmaceutical company Servier for additional information on all unpublished studies were unsuccessful.
Authors' conclusions: Agomelatine did not seem to provide a significant advantage in efficacy over other antidepressive agents for the acute-phase treatment of major depression. Agomelatine was better tolerated than paroxetine and venlafaxine in terms of overall side effects, and fewer participants treated with agomelatine dropped out of the trials due to side effects compared to sertraline and venlafaxine, but data were limited because the number of included studies was small. We found evidence that compared agomelatine with only a small number of other active antidepressive agents, and there were only a few trials for each comparison, which limits the generalisability of the results. Moreover, the overall methodological quality of the studies was low, and, therefore, no firm conclusions can be drawn concerning the efficacy and tolerability of agomelatine.
Conflict of interest statement
Giuseppe Guaiana: none known Sumeet Gupta: none known Debbie Chiodo: none known Simon JC Davies: none known Katja Haederle: none known Markus Koesters: none known
Figures
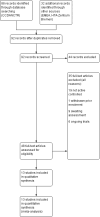

























































Update of
References
References to studies included in this review
CAGO2303 {unpublished data only}
-
- Novartis Pharmaceuticals. A placebo‐ and paroxetine‐controlled study of the efficacy, safety and tolerability of agomelatine (25 or 50 mg) in the treatment of major depressive disorder (MDD) [NCT00463242]. ClinicalTrials.gov [www.clinicaltrials.gov] [accessed 10 November 2012] 2009.
-
- Novartis Pharmaceuticals. An 8‐week, multicenter, randomized, double‐blind, placebo‐and paroxetine‐controlled study of the efficacy, safety and tolerability of agomelatine 25 or 50 mg given once daily in the treatment of Major Depressive Disorder (MDD). http://www.novctrd.com/ctrdWebApp/clinicaltrialrepository/displayFile.do... [accessed 10 November 2012] 2009.
CL3‐022 {unpublished data only}
-
- European Medicines Agency. CHMP Assessment report for Thymanax [Procedure No. EMEA/H/C/000916, Doc. Ref. EMEA/97539/2009].http://www.ema.europa.eu/docs/en_GB/document_library/EPAR_‐_Public_assessment_r.... [Accessed 22 October 2012] 2008.
-
- European Medicines Agency. CHMP Assessment report for Thymanax [Procedure No. EMEA/H/C/000916, Doc. Ref. EMEA/97539/2009].http://www.ema.europa.eu/docs/en_GB/document_library/EPAR_‐_Public_assessment_r.... [Accessed 25 October 2012] 2009.
-
- European Medicines Agency. CHMP Assessment report for Valdoxan [Procedure No. EMEA/H/C/656, Doc. Ref. EMEA/CHMP/87018/2006].http://www.ema.europa.eu/docs/en_GB/document_library/EPAR_‐_Public_assessment_r.... [Accessed 22 January 2013] 2006.
CL3‐023 {unpublished data only}
-
- European Medicines Agency. CHMP Assessment report for Thymanax [Procedure No. EMEA/H/C/000916, Doc. Ref. EMEA/97539/2009].http://www.ema.europa.eu/docs/en_GB/document_library/EPAR_‐_Public_assessment_r.... [Accessed 22 October 2012] 2008.
-
- European Medicines Agency. CHMP Assessment report for Thymanax [Procedure No. EMEA/H/C/000916, Doc. Ref. EMEA/97539/2009].http://www.ema.europa.eu/docs/en_GB/document_library/EPAR_‐_Public_assessment_r.... [Accessed 25 October 2012] 2009.
-
- European Medicines Agency. CHMP Assessment report for Valdoxan [Procedure No. EMEA/H/C/656, Doc. Ref. EMEA/CHMP/87018/2006].http://www.ema.europa.eu/docs/en_GB/document_library/EPAR_‐_Public_assessment_r.... [Accessed 22 January 2013] 2006.
CL3‐024 {unpublished data only}
-
- European Medicines Agency. CHMP Assessment report for Thymanax [Procedure No. EMEA/H/C/000916, Doc. Ref. EMEA/97539/2009].http://www.ema.europa.eu/docs/en_GB/document_library/EPAR_‐_Public_assessment_r.... [Accessed 22 October 2012] 2008.
-
- European Medicines Agency. CHMP Assessment report for Thymanax [Procedure No. EMEA/H/C/000916, Doc. Ref. EMEA/97539/2009].http://www.ema.europa.eu/docs/en_GB/document_library/EPAR_‐_Public_assessment_r.... [Accessed 25 October 2012] 2009.
-
- European Medicines Agency. CHMP Assessment report for Valdoxan [Procedure No. EMEA/H/C/656, Doc. Ref. EMEA/CHMP/87018/2006].http://www.ema.europa.eu/docs/en_GB/document_library/EPAR_‐_Public_assessment_r.... [Accessed 22 January 2013] 2006.
Corruble 2013 {published data only}
-
- Corruble E, Belaidi C, Goodwin GM. Agomelatine versus escitalopram in major depressive disorders. European Psychiatry [abstracts from the 19th European Congress of Psychiatry, EPA 2011 Mar 12‐15; Vienna, Austria] 2011;26(S1):619.
-
- Corruble E, Bodinat C, Belaidi C, Goodwin GM. Efficacy of agomelatine and escitalopram on depression, subjective sleep and emotional experiences in patients with major depressive disorder: a 24‐wk randomized, controlled, double‐blind trial. International Journal of Neuropsychopharmacology 2013:1‐16 [epub ahead of print]. - PubMed
Hale 2010 {published data only (unpublished sought but not used)}
-
- Hale A. Efficacy and safety of agomelatine (25 mg/day with potential adjustment to 50 mg) given orally for eight weeks in out‐patients with severe major depressive disorder: a randomised double‐blind, parallel groups, international study versus selective serotonin reuptake inhibitor (SSRI) with a double‐blind extension period of 16 weeks. Controlled‐Trials.com 2008 (http://www.controlled‐trials.com/ISRCTN19313268/servier).
-
- Hale A, Corral RM, Mencacci C, Ruiz JS, Severo CA, Gentil V. Superior antidepressant efficacy results of agomelatine versus fluoxetine in severe MDD patients: a randomized, double‐blind study. International Clinical Psychopharmacology 2010;25(6):305‐14. - PubMed
Kasper 2010 {published data only (unpublished sought but not used)}
-
- Kasper S, Hajak G, Wulff K, Hoogendijk WJG, Montejo AL, Smeraldi E, et al. Efficacy of the novel antidepressant agomelatine on the circadian rest‐activity cycle and depressive and anxiety symptoms in patients with major depressive disorder: A randomized, double‐blind comparison with sertraline [ISRCTN49376288]. Journal of Clinical Psychiatry 2010;71(2):109‐20. - PubMed
Kennedy 2008 {published data only (unpublished sought but not used)}
-
- Kennedy SH, Rizvi S, Fulton K, Rasmussen J. A double‐blind comparison of sexual functioning, antidepressant efficacy, and tolerability between agomelatine and venlafaxine XR. Journal of Clinical Psychopharmacology 2008;28(3):329‐33. - PubMed
Lemoine 2007 {published data only (unpublished sought but not used)}
-
- Lemoine P, Guilleminault C, Alvarez E. Improvement in subjective sleep in major depressive disorder with a novel antidepressant, agomelatine: randomized, double‐blind comparison with venlafaxine. Journal of Clinical Psychiatry 2007;68(11):1723‐32. - PubMed
Loo 2002a {published data only}
-
- Loo H, Hale A, D'haenen H. Determination of the dose of agomelatine, a melatoninergic agonist and selective 5‐HT(2C) antagonist, in the treatment of major depressive disorder: a placebo‐controlled dose range study. International Clinical Psychopharmacology 2002;17(5):239‐47. - PubMed
Martinotti 2012 {published data only}
-
- Martinotti G, Sepede G, Nicola M, Iorio G, Gambi F, Risio L, et al. Agomelatine versus venlafaxine in the treatment of anhedonia in major depressive subjects: A pilot study [oral presentation]. European Psychiatry [abstracts of the 20th European Congress of Psychiatry, EPA. 2012 Mar 3‐6; Prague Czech Republic] 2012;27(Suppl 1):O‐35.
-
- Martinotti G, Sepede G, Gambi F, Iorio G, Berardis D, Nicola M, et al. Agomelatine versus venlafaxine XR in the treatment of anhedonia in major depressive disorder: A pilot study. Journal of Clinical Psychopharmacology 2012;32(4):487‐91. - PubMed
Quera‐Salva 2011 {published data only (unpublished sought but not used)}
-
- Quera‐Salva M‐A, Hajak G, Philip P, Montplaisir J, Keufer‐Le Gall S, Laredo J, et al. Comparison of agomelatine and escitalopram on nighttime sleep and daytime condition and efficacy in major depressive disorder patients. International Clinical Psychopharmacology 2011;26(5):252‐62. - PubMed
-
- Quera‐Salva M‐A, Servier Laboratories. Effects of agomelatine on sleep electroencephalogram parameters compared to selective serotonin reuptake inhibitors in patients with major depressive disorder: a six‐week randomised, double‐blind parallel group study versus comparator, followed by a double‐blind optional treatment extension period up to six months [ISRCTN44737909; CL3‐20098‐056; EUCTR2006‐004716‐48]. Controlled‐Trials.com [www.controlled‐trials.com] 2007.
Shu 2013 {published data only}
-
- Shu L, Zhang Y, Servier Laboratories. Efficacy and safety of agomelatine with flexible dose (25 mg/day with potential adjustment at 50 mg) given orally for 8 weeks in out‐patients with Major Depressive Disorder. A randomised flexible dose double‐blind international multicentric study with parallel groups, versus fluoxetine (20 mg/day with potential adjustment at 40 mg) [ChiCTR‐TRC‐11001668]. Chinese Clinical Trial Registry [http://www.chictr.org/en] 2011.
References to studies excluded from this review
CAGO2301 {published data only}
-
- Novartis Pharmaceuticals. An 8‐week, randomized, double‐blind, placebo‐controlled, parallel‐group, multi‐center study of the efficacy and safety of agomelatine 0.5 mg and 1 mg sublingual tablets administered once daily in patients with major depressive disorder (MDD) [NCT01110889; CAGO178C2301]. ClinicalTrials.gov [www.clinicaltrials.gov] 2010.
CAGO2302 {published data only}
-
- Novartis Pharmaceuticals. A 8‐week, randomized, double‐blind, placebo‐controlled, parallel‐group, multi‐center study of the efficacy and safety of agomelatine 0.5 mg and 1 mg sublingual tablets administered once daily in patients with major depressive disorder (MDD) [NCT01110902; CAGO178C2302]. ClinicalTrials.gov [www.clinicaltrials.gov] 2010.
CAGO2304 {published data only}
-
- Novartis Pharmaceuticals. A 52‐week, randomized, double‐blind, placebo‐controlled, multi‐center, parallel‐group study of the long‐term efficacy, tolerability and safety of agomelatine 25 and 50 mg in the prevention of relapse of major depressive disorder (MDD) following open‐label treatment of 16‐24 weeks [NCT00467402; CAGO178A2304]. ClinicalTrials.gov [www.clinicaltrials.gov] 2007.
CL2‐009 {published data only}
-
- Servier Laboratories. Efficacy and safety of 3 doses (0.25, 0.5 and 1mg/day) of agomelatine sublingual administration over an 8‐week treatment period, in out‐patients with Major Depressive Disorder. An 8‐week randomised, double‐blind, fixed dose, international multicentre, placebo‐controlled study with parallel groups, followed by an extension double‐blind treatment period of 16 weeks [CL2‐90098‐009; EUCTR2009‐014045‐92]. EU Clinical Trials Register [.www.clinicaltrialsregister.eu] 2009.
CL3‐021 {published data only}
-
- European Medicines Agency. CHMP Assessment report for Thymanax [Procedure No. EMEA/H/C/000916, Doc. Ref. EMEA/97539/2009].http://www.ema.europa.eu/docs/en_GB/document_library/EPAR_‐_Public_assessment_r... [Accessed 22 October 2012] 2008.
-
- European Medicines Agency. CHMP Assessment report for Thymanax [Procedure No. EMEA/H/C/000916, Doc. Ref. EMEA/97539/2009].http://www.ema.europa.eu/docs/en_GB/document_library/EPAR_‐_Public_assessment_r.... [Accessed 25 October 2012] 2009.
CL3‐025 {published data only}
-
- European Medicines Agency. CHMP Assessment report for Thymanax [Procedure No. EMEA/H/C/000916, Doc. Ref. EMEA/97539/2009].http://www.ema.europa.eu/docs/en_GB/document_library/EPAR_‐_Public_assessment_r.... [Accessed 22 October 2012] 2008.
-
- European Medicines Agency. CHMP Assessment report for Thymanax [Procedure No. EMEA/H/C/000916, Doc. Ref. EMEA/97539/2009].http://www.ema.europa.eu/docs/en_GB/document_library/EPAR_‐_Public_assessment_r.... [Accessed 25 October 2012] 2009.
CL3‐026 {published data only}
-
- European Medicines Agency. CHMP Assessment report for Thymanax [Procedure No. EMEA/H/C/000916, Doc. Ref. EMEA/97539/2009].http://www.ema.europa.eu/docs/en_GB/document_library/EPAR_‐_Public_assessment_r.... [Accessed 22 October 2012] 2008.
-
- European Medicines Agency. CHMP Assessment report for Thymanax [Procedure No. EMEA/H/C/000916, Doc. Ref. EMEA/97539/2009].http://www.ema.europa.eu/docs/en_GB/document_library/EPAR_‐_Public_assessment_r.... [Accessed 25 October 2012] 2009.
Corral 2009 {published data only}
-
- Corral RM, Servier Laboratories. Efficacy and safety of three dose regimens of agomelatine (10, 25, 25 ‐ 50 mg) versus placebo given once a day for 6 weeks in out‐patients suffering from moderate to severe major depressive disorder: a 6‐week randomised, double‐blind, placebo‐controlled, parallel groups study followed by a double‐blind optional 18‐week extension period [ISRCTN10845256; CL3‐20098‐069; EUCTR2009‐011238‐84]. Controlled‐Trials.com [www.controlled‐trials.com] 2009.
Goodwin 2009 {published data only}
-
- Goodwin GM, Emsley R, Rembry S, Rouillon F, Agomelatine Study Group. Agomelatine prevents relapse in patients with major depressive disorder without evidence of a discontinuation syndrome: a 24‐week randomized, double‐blind, placebo‐controlled trial [ISRCTN53193024]. Journal of Clinical Psychiatry 2009;70(8):1128‐37. - PubMed
Heun 2013 {published data only}
-
- Heun R, Ahokas A, Boyer P, Gimenez‐Montesinos N, Pontes‐Soares F, Olivier V. The efficacy of agomelatine in elderly patients with recurrent major depressive disorder: a placebo‐controlled study. Journal of Clinical Psychiatry 2013;74(6):587‐94. - PubMed
-
- Heun R, Servier Laboratories. Efficacy and safety of agomelatine oral administration (25 to 50 mg/day) in elderly patients suffering from major depressive disorder: an 8‐week, randomised, double‐blind, flexible‐dose, parallel groups, placebo‐controlled, international, multicentre study followed by an extension double‐blind treatment period of 16 weeks [ISRCTN57507360; CL3‐20098‐070; EUCTR2009‐011795‐29]. Controlled‐Trials.com [www.controlled‐trials.com] 2010.
Kennedy 2006 {published data only}
-
- Kennedy SH, Emsley R. Placebo‐controlled trial of agomelatine in the treatment of major depressive disorder. European Neuropsychopharmacology 2006;16(2):93‐100. - PubMed
Loo 2002b {published data only}
-
- Loo H, Dalery J, Macher JP, Payen A. Pilot study comparing in blind the therapeutic effect of two doses of agomelatine, melatoninergic agonist and selective 5ht2c receptors antagonist, in the treatment of major depressive disorders. L'Encephale 2002;28(4):356‐62. - PubMed
Olie 2007 {published data only}
-
- Olie JP, Kasper S. Efficacy of agomelatine, a MT1/MT2 receptor agonist with 5‐HT2C antagonistic properties, in major depressive disorder. International Journal of Neuropsychopharmacology 2007;10(5):661‐73. - PubMed
Rouillon 2008 {published data only}
-
- Rouillon F, Servier Laboratories. Efficacy and safety of two doses of S 90098 (1 and 2 mg/day), sublingual formulation for 8 weeks in out‐patients with major depressive disorder: An 8‐week randomised, double‐blind, fixed dose, international, multicentre, placebo‐controlled study with parallel groups, followed by an extension double‐blind treatment period for 16 weeks [ISRCTN38378163; CL2‐90098‐005]. Controlled‐Trials.com [www.controlled‐trials.com] 2008.
Saletu 2011 {published data only}
-
- Saletu M, Saletu‐Zyhlarz GM, Anderer P, Rosales‐Rodriguez S, Saletu B. On the acute effect of agomelatine on sleep and awakening in major depression: controlled polysomnographic and psychometric studies [abstract]. Somnologie [abstracts of the 19th Jahrestagung der DGSM; 2011 Nov 10‐12; Mannheim Germany] 2011.
Save 2011 {published data only}
-
- Save D, Precise Chemipharma Pvt Ltd. A multicentric, open‐label, randomized, comparative, parallel‐group, active‐controlled Phase III clinical trial of the efficacy and safety of agomelatine oral tablets in patients with major depressive disorder [CTRI/2011/08/001946]. Clinical Trials Registry ‐ India [http://ctri.nic.in] 2011.
Serfaty 2010 {published data only}
-
- Serfaty MA, Osborne D, Buszewicz MJ, Blizard R, Raven PW. A randomized double‐blind placebo‐controlled trial of treatment as usual plus exogenous slow‐release melatonin (6 mg) or placebo for sleep disturbance and depressed mood. International Clinical Psychopharmacology 2010;25(3):132‐42. - PubMed
Stahl 2010 {published data only}
-
- Stahl SM, Fava M, Trivedi MH, Caputo A, Shah A, Post A. Agomelatine in the treatment of major depressive disorder: an 8‐week, multicenter, randomized, placebo‐controlled trial [NCT00411242]. Journal of Clinical Psychiatry 2010;71(5):616‐26. - PubMed
Tseng 2007 {published data only}
-
- Tseng M‐C. The effect of agomelatine or fluoxentine on heart rate variability in patients with major depressive disorder [NCT00451490]. ClinicalTrials.gov [www.clinicaltrials.gov] 2007.
Zajecka 2010 {published data only}
-
- Zajecka J, Schatzberg A, Stahl S, Shah A, Caputo A, Post A. Efficacy and safety of agomelatine in the treatment of major depressive disorder: a multicenter, randomized, double‐blind, placebo‐controlled trial. Journal of Clinical Psychopharmacology 2010;30(2):135‐44. - PubMed
References to studies awaiting assessment
CL3‐027 {published data only}
-
- European Medicines Agency. CHMP Assessment report for Valdoxan [Procedure No. EMEA/H/C/656, Doc. Ref. EMEA/CHMP/87018/2006].http://www.ema.europa.eu/docs/en_GB/document_library/EPAR_‐_Public_assessment_r.... [Accessed 22 January 2013] 2006.
CL3‐048 {published data only}
-
- Bougerol T, Servier Laboratories. Efficacy of agomelatine given orally on the quality of remission in elderly depressed patients, after a 12‐week treatment period. A randomised, double‐blind, flexible‐dose international multicentre study with parallel groups versus SSRI drug. Twelve‐week treatment plus optional continuation for 12 weeks [ISRCTN68222771; CL3‐20098‐048; EUCTR2005‐002388‐95]. Controlled‐Trials.com [www.controlled‐trials.com] 2006.
CL3‐062 {published data only}
-
- Marey C, Servier Laboratories. Evaluation of efficacy and clinical benefit of agomelatine in patients with major depressive disorder compared to serotonin‐norepinephrine reuptake inhibitor (SNRI) [ISRCTN96725312; CL3‐20098‐062; EUCTR2008‐004642‐92]. Controlled‐Trials.com [www.controlled‐trials.com] 2009.
CL3‐073 {published data only}
-
- Lejoyeux M, Servier Laboratories. Initiation of agomelatine after antidepressant treatment in outpatients suffering major depressive disorder [ISRCTN97599615; CL3‐20098‐073; EUCTR2010‐019556‐44]. Controlled‐Trials.com [www.controlled‐trials.com] 2010.
CRSC11003 {published data only}
-
- Cadila Pharmaceuticals Limited. A randomized, multi‐center, double blind, controlled, comparative study of the efficacy and safety of agomelatine 25 mg (or 50 mg) and paroxetine 20mg (or 30 mg) in patients with major depressive disorder (MDD) [CTRI/2012/03/002480; CRSC11003]. Clinical Trials Registry ‐ India [http://ctri.nic.in] 2012.
CTRI/2011/04/001659 {published data only}
-
- Borgharkar S, Sun Pharmaceutical Industries Ltd. Evaluation of efficacy and safety of agomelatine versus venlafaxine ER in the treatment of major depressive disorder. Clinical Trials Registry ‐ India [http://ctri.nic.in] 2011.
Karaiskos 2013 {published data only}
-
- Karaiskos D, Tzavellas E, Ilias I, Liappas I, Paparrigopoulos T. Agomelatine and sertraline for the treatment of depression in type 2 diabetes mellitus. International Journal of Clinical Practice 2013;67(3):257‐60. - PubMed
Montgomery 2004 {published data only}
-
- Montgomery SA, Kennedy SH, Burrows GD, Lejoyeux M, Hindmarch I. Absence of discontinuation symptoms with agomelatine and occurrence of discontinuation symptoms with paroxetine: a randomized, double‐blind, placebo‐controlled discontinuation. International Clinical Psychopharmacology 2004;19(5):271‐80. - PubMed
Vasile 2011 {published data only}
-
- Vasile D, Vasiliu O, Vasile ML, Terpan M, Ojog DG. Agomelatine versus selective serotoninergic reuptake inhibitors in major depressive disorder and comorbid diabetes mellitus [conference abstract]. European Neuropsychopharmacology [abstracts from the 24th Congress of the European College of Neuropsychopharmacology, ECNP 2011 Paris France, 3‐7 Sept] 2011;21(Suppl 3):S383‐4.
References to ongoing studies
CL3‐060 {published data only}
-
- Servier Laboratories. Effects of agomelatine versus escitalopram on emotional experiences in outpatients suffering from major depressive disorder. An exploratory, randomised, double‐blind, international, multicentre study with parallel groups: agomelatine (25 to 50 mg/day) versus escitalopram (10 to 20 mg/day) over a 6‐month period [EUCTR2011‐005320‐17‐GB; CL3‐20098‐060]. EU Clinical Trials Register [www.clinicaltrialsregister.eu] [Accessed 20 September 2013] 2012.
CL3‐074 {published data only}
-
- Shah A, Rajarshi M. Efficacy and safety of agomelatine with flexible dose (25 mg/day with blinded adjustment at 50 mg) given orally for 8 weeks in Indian outpatients with major depressive disorder. A randomised double‐blind national multicentric study with parallel groups, versus sertraline (50 mg/day with blinded potential adjustment at 100 mg) [CTRI/2010/091/006081; CL3‐20098‐074]. Clinical Trials Registry ‐ India [http://ctri.nic.in] [Accessed 20 September 2013] 2012.
CL3‐083 {published data only}
-
- Udristoiu T, Servier Laboratories. Early effect of agomelatine on general interest in outpatients suffering major depressive disorder: a parallel group, randomised, double‐blind, multicentre study [ISRCTN28327843; CL3‐20098‐083]. Controlled‐Trials.com [www.controlled‐trials.com] [Accessed 20 September 2013] 2011.
GENRAS {published data only}
-
- Medizinische Universität Wien Univ Klinik f Psychiatrie und Psychotherapie. GENRAS (GENetics of Response to Agomelatine vs. EScitalopram) [EUCTR2010‐019423‐61‐AT]. EU Clinical Trials Register [www.clinicaltrialsregister.eu] [Accessed 20 September 2013] 2010.
Lundbeck 2011 {published data only}
-
- H Lundbeck A/S. A randomised, double‐blind, parallel‐group, active‐controlled, flexible dose study evaluating the effects of Lu AA21004 versus agomelatine in adult patients suffering from major depressive disorder with inadequate response to antidepressant treatment [NCT01488071; EUCTR2011‐002362‐21]. ClinicalTrials.gov [www.clinicaltrials.gov] [Accessed 20 September 2013] 2011.
NCT01483053 {published data only}
-
- Baker IDI Heart and Diabetes Institute. A randomised trial investigating the cardiovascular effects of agomelatine and escitalopram in patients with major depressive disorder [NCT01483053]. ClinicalTrials.gov [www.clinicaltrials.gov] [Accessed 20 September 2013] 2011.
Additional references
AHRQ 2007
-
- Agency for Healthcare Research and Quality. Comparative effectiveness of second‐generation antidepressants in the pharmacologic treatment of adult depression. http://www.effectivehealthcare.ahrq.gov/ehc/products/7/61/Antidepressant... (accessed 9 April 2010). - PubMed
Als‐Nielsen 2003
-
- Als‐Nielsen B, Chen W, Gluud C, Kjaegard LL. Association of funding and conclusions in randomized drug trials. JAMA 2003;290:921‐8. - PubMed
Altman 1996
APA 1980
-
- American Psychiatric Association. Diagnostic and Statistical Manual of Mental Disorder (DSM‐III), 3rd edition. American Psychiatric Press, 1980.
APA 1987
-
- American Psychiatric Association. Diagnostic and Statistical Manual of Mental Disorders (DSM‐III‐R), 3rd edition revised. American Psychiatric Publishing, 1987.
APA 1994
-
- American Psychiatric Association. Diagnostic and Statistical Manual of Mental Disorders (DSM‐IV), 4th edition. American Psychiatric Publishing, 2004.
APA 2000
-
- American Psychiatric Association. Diagnostic and Statistical Manual of Mental Disorders (DSM‐IV‐TR) 4th Edition ‐ revised. American Psychiatric Publishing, 2000.
APA 2000a
-
- American Psychiatric Association. Practice guideline for the treatment of patients with major depressive disorder (revision). American Journal of Psychiatry 2000;157:1‐45. - PubMed
Barbui 2004
-
- Barbui C, Cipriani A, Brambilla P, Hotopf M. "Wish bias" in antidepressant drug trials?. Journal of Clinical Psychopharmacology 2004;2:126‐30. - PubMed
Beck 1987
-
- Beck AT, Steer R. Beck Depression Inventory. Psychological Corporation, 1987.
Bhandari 2004
Boissel 1999
-
- Boissel JP, Cucherat M, Li W, Chatellier G, Gueyffier F, Buyse M, et al. The problem of therapeutic efficacy indices. Comparison of the indices and their use. Therapie 1999;54:405‐11. - PubMed
Bollini 1999
-
- Bollini P, Pampallona S, Tibaldi G, Kupelnick B, Munizza C. Effectiveness of antidepressants. Meta‐analysis of dose‐effect relationships in randomised clinical trials. British Journal of Psychiatry 1999;174:297‐303. - PubMed
Buchkowsky 2004
-
- Buchkowsky SS, Jewesson PJ. Industry sponsorship and authorship of clinical trials over 20 years. The Annals of Pharmacotherapy 2004;38:579‐85. - PubMed
Cipriani 2009
-
- Cipriani A, Furukawa TA, Salanti G, Geddes JR, Higgins JPT, Churchill R, et al. Comparative efficacy and acceptability of 12 new‐generation antidepressants: a multiple‐treatments meta‐analysis. Lancet 2009;373:746‐58. - PubMed
Clayton 2006
-
- Clayton AH, Montejo AL. Major depressive disorder, antidepressants,and sexual dysfunction. Journal of Clinical Psychiatry 2006;67(Suppl 6):33‐7. - PubMed
Cohen 1960
-
- Cohen J. A coefficient of agreement for nominal scales. Educational and Psychological Measurement 1960;20:37‐46.
Deeks 2000
-
- Deeks J. Issues in the selection for meta‐analyses of binary data. Proceedings of the 8th International Cochrane Colloquium; Cape Town, South Africa. 2000 Oct 25‐28th.
Dolder 2008
-
- Dolder CR, Nelson M, Snider M. Agomelatine treatment of major depressive disorder. The Annals of Pharmacotherapy 2008;421:822‐31. - PubMed
Dording 2002
-
- Dording CM, Mischoulon D, Petersen TJ, Kornbluh R, Gordon J, Nierenberg AA, et al. The pharmacologic management of SSRI‐induced side effects: a survey of psychiatrists. Annals of Clinical Psychiatry 2002;14:143‐7. - PubMed
Elbourne 2002
-
- Elbourne DR, Altman DG, Higgins JP, Curtin F, Worthington HV, Vail A. Meta‐analyses involving cross‐over trials: methodological issues. International Journal of Epidemiology 2002;31:140‐9. - PubMed
EMEA 2009
-
- European Medicines Agency. CHMP Assessment report for Thymanax [Procedure No. EMEA/H/C/000916, Doc. Ref. EMEA/97539/2009].http://www.ema.europa.eu/docs/en_GB/document_library/EPAR_‐_Public_assessment_r.... (Accessed 25 October 2012) 2009.
Feighner 1972
-
- Feighner JP, Robins E, Guze SB, Woodruff RA, Winokur G, Munoz R. Diagnostic criteria for use in psychiatric research. Archives of General Psychiatry 1972;26:57‐63. - PubMed
Fournier 2010
Furukawa 2005
-
- Furukawa TA, Cipriani A, Barbui C, Brambilla P, Watanabe N. Imputing response rates from means and standard deviations in meta‐analysis. International Clinical Psychopharmacology 2005;20:49‐52. - PubMed
Furukawa 2006
-
- Furukawa TA, Barbui C, Cipriani A, Brambilla P, Watanabe N. Imputing missing standard deviations in meta‐analyses can provide accurate results. Journal of Clinical Epidemiology 2000;59:7‐10. - PubMed
Guy 1976
-
- Guy W (editor). ECDEU Assessment Manual for Psychopharmacology. US Department of Health, Education and Welfare, 1976.
Guyatt 2008
Hamilton 1960
Hansen 2005
-
- Hansen RA, Gartlhner G, Lohr KN, Gaynes BN, Carey TS. Efficacy and safety of second‐generation antidepressants in the treatment of major depressive disorder. Annals of Internal Medicine 2005;143:415‐26. - PubMed
Higgins 2011
-
- Higgins JPT, Green S (editors). Cochrane Handbook for Systematic Reviews of Interventions Version 5.1.0 [updated March 2011]. The Cochrane Collaboration, 2011. Available from www.cochrane‐handbook.org. Chichester: John Wiley & Sons.
Howland 2011
-
- Howland RH. Publication bias and outcome reporting bias: agomelatine as a case example. Journal of Psychosocial Nursing and Mental Health Services 2011;49:11‐4. - PubMed
Kasper 2008
-
- Kasper S, Laigle L, Baylè F. Superior antidepressant efficacy of agomelatine vs sertraline: A randomized, double‐blind study. Poster presentation 21st ECNP Congress, Barcelona, Spain. 30 August ‐ 3 September 2008.
Kasper 2013
-
- Kasper S, Corruble E, Hale A, Lemoine P, Montgomery SA, Quera‐Salva MA. Antidepressant efficacy of agomelatine versus SSRI/SNRI: results from a pooled analysis of head‐to‐head studies without a placebo control. International Clinical Psychopharmacology 2013;28:12‐9. - PubMed
Kessler 2003
-
- Kessler RC, Berglund P, Demler O, Jin R, Koretz D, Merikangas KR, et al. National Comorbidity Survey Replication. The epidemiology of major depressive disorder: results from the National Comorbidity Survey Replication (NCS‐R). JAMA 2003;289:3095‐105. - PubMed
Khan 2003
-
- Khan A, Khan SR, Walens G, Kolts R, Giller EL. Frequency of positive studies among fixed and flexible dose antidepressant clinical trials: an analysis of the food and drug administration summary basis of approval reports. Neuropsychopharmacology 2003;28:552‐7. - PubMed
Koesters 2013
-
- Koesters M, Guaiana G, Cipriani A, Becker T, Barbui C. Agomelatine efficacy and acceptability revisited: systematic review and meta‐analysis of published and unpublished randomised trials. The British Journal of Psychiatry 2013;203(3):179‐87. - PubMed
Lam 2009
-
- Lam RW, Kennedy SH, Grigoriadis S, McIntyre RS, Milev R, Ramasubbu R, et al. Canadian Network for Mood and Anxiety Treatments (CANMAT). Canadian Network for Mood and Anxiety Treatments (CANMAT) clinical guidelines for the management of major depressive disorder in adults. III Pharmacotherapy. Journal of Affective Disorder 2009;117 Suppl 1:26‐43. - PubMed
Lexchin 2003
Lundh 2012
Middleton 2001
-
- Middleton N, Gunnell D, Whitley E, Dorling D, Frankel S. Secular trends in antidepressants prescribing in the UK, 1975‐98. Journal of Public Health Medicine 2001;23:262‐7. - PubMed
Millan 2003
-
- Millan MG, Gobert A, Lejeune F, Dekeyne A, Newman‐Tancredi A, Pasteau V, et al. The novel melatonin agonist agomelatine (S20098) is an antagonist at 5‐hydroxytryptamine2C receptors, blockade of which enhances the activity of frontocortical dopaminergic and adrenergic pathways. Journal of Pharmacology and Experimental Therapeutics 2003;306:954‐64. - PubMed
Montgomery 1979
-
- Montgomery SA, Asberg M. A new depression scale designed to be sensitive to change. British Journal of Psychiatry 1979;134:382‐9. - PubMed
Müller 2003
-
- Müller MJ, Himmerich H, Kienzle B, Szegedi A. Differentiating moderate and severe depression using the Montgomery Asberg depression rating scale (MADRS). Journal of Affective Disorders 2003;77(3):255‐60. - PubMed
NICE 2004
-
- National Institute of Clinical Excellence. Depression: management of depression in primary and secondary care. National Institute of Clinical Excellence, 2004.
Novartis 2012
-
- Novartis. Annual report 2011. http://www.novartis.com/downloads/investors/reports/novartis‐annual‐repo... [Accessed 20 June 2013] 2012.
Oxman 1992
-
- Oxman AD, Guyatt GH. A consumer's guide to subgroup analyses. Annals of Internal Medicine 1992;116(1):78‐84. - PubMed
Popoli 2009
-
- Popoli M. Agomelatine: innovative pharmacological approach in depression. CNS Drugs 2009;23 Suppl 2:27‐34. - PubMed
Prescrire 2013
-
- Agomelatine: a review of adverse effects. Prescrire International 2013;136:70‐1. - PubMed
Radloff 1977
-
- Radloff LS. The CES‐D scale: a self‐report depression scale for research in the general population. Applied Psychological Measurement 1977;1:385‐401.
Rosenbaum 2010
-
- Rosenbaum SE, Glenton C, Oxman AD. Summary‐of‐findings tables in Cochrane reviews improved understanding and rapid retrieval of key information. Journal of Clinical Epidemiology 2010;63:620‐6. - PubMed
San 2008
-
- San L, Arranz B. Agomelatine: a novel mechanism of antidepressant action involving the melatonergic and the serotonergic system. European Psychiatry 2008;23:396‐402. - PubMed
Servier 2013
-
- Servier Laboratories Ltd. Agomelatine (Valdoxan): monitor liver function and do not use in people with high transaminase levels (>3 x ULN) or ≥ 75 years. http://www.servier.co.uk/pdfs/direct‐healthcare‐professional‐communicati... [Accessed 20 September 2013] 2013.
Singh 2011
-
- Singh SP, Singh V, Kar N. Efficacy of agomelatine in major depressive disorder: meta‐analysis and appraisal. International Journal of Neuropsychopharmacology 2011;23:1‐12. - PubMed
Spitzer 1978
-
- Spitzer RL, Robins E. Research diagnostic criteria: rationale and reliability. Archives of General Psychiatry 1978;35:773‐82. - PubMed
Taylor 2009
-
- Taylor D, Paton C, Kapur S. The Maudesley Prescribing Guidelines. The Maudsley Prescribing Guidelines. Informa Healthcare, 2009.
WHO 1978
-
- World Health Organization. The Ninth Revision of the International Classification of Diseases and Related Health Problems (ICD‐9). World Health Organization, 1978.
WHO 1992
-
- World Health Organization. The Tenth Revision of the International Classification of Diseases and Related Health Problems (ICD‐10). World Health Organization, 1992.
WHO 2008
-
- World Health Organization. The Global Burden of Disease. 2004 update. World Health Organization, 2008.
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

