Association between pediatric clinical trials and global burden of disease
- PMID: 24344112
- PMCID: PMC3876184
- DOI: 10.1542/peds.2013-2567
Association between pediatric clinical trials and global burden of disease
Abstract
Background: The allocation of research resources should favor conditions responsible for the greatest disease burden. This is particularly important in pediatric populations, which have been underrepresented in clinical research. Our aim was to measure the association between the focus of pediatric clinical trials and burden of disease and to identify neglected clinical domains.
Methods: We performed a cross-sectional study of clinical trials by using trial records in ClinicalTrials.gov. All trials started in 2006 or after and studying patient-level interventions in pediatric populations were included. Age-specific measures of disease burden were obtained for 21 separate conditions for high-, middle-, and low-income countries. We measured the correlation between number of pediatric clinical trials and disease burden for each condition.
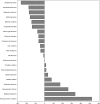
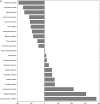
Results: Neuropsychiatric conditions and infectious diseases were the most studied conditions globally in terms of number of trials (874 and 847 trials, respectively), while intentional injuries (5 trials) and maternal conditions (4 trials) were the least studied. Clinical trials were only moderately correlated with global disease burden (r = 0.58, P = .006). Correlations were also moderate within each of the country income levels, but lowest in low-income countries (r = .47, P = .03). Globally, the conditions most understudied relative to disease burden were injuries (-260 trials for unintentional injuries and -160 trials for intentional injuries), nutritional deficiencies (-175 trials), and respiratory infections (-171 trials).
Conclusions: Pediatric clinical trial activity is only moderately associated with pediatric burden of disease, and least associated in low-income countries. The mismatch between clinical trials and disease burden identifies key clinical areas for focus and investment.
Keywords: burden of disease; clinical trials; pediatric research.
Figures


References
-
- Cohen E, Uleryk E, Jasuja M, Parkin PC. An absence of pediatric randomized controlled trials in general medical journals, 1985–2004. J Clin Epidemiol. 2007;60(2):118–123 - PubMed
-
- Cohen E, Goldman RD, Ragone A, et al. Child vs adult randomized controlled trials in specialist journals: a citation analysis of trends, 1985–2005. Arch Pediatr Adolesc Med. 2010;164(3):283–288 - PubMed
-
- Martinez-Castaldi C, Silverstein M, Bauchner H. Child versus adult research: the gap in high-quality study design. Pediatrics. 2008;122(1):52–57 - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

