KNL1 facilitates phosphorylation of outer kinetochore proteins by promoting Aurora B kinase activity
- PMID: 24344188
- PMCID: PMC3871439
- DOI: 10.1083/jcb.201306054
KNL1 facilitates phosphorylation of outer kinetochore proteins by promoting Aurora B kinase activity
Abstract
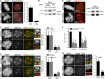
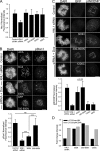
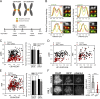
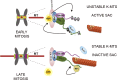
Aurora B kinase phosphorylates kinetochore proteins during early mitosis, increasing kinetochore–microtubule (MT) turnover and preventing premature stabilization of kinetochore–MT attachments. Phosphorylation of kinetochore proteins during late mitosis is low, promoting attachment stabilization, which is required for anaphase onset. The kinetochore protein KNL1 recruits Aurora B–counteracting phosphatases and the Aurora B–targeting factor Bub1, yet the consequences of KNL1 depletion on Aurora B phospho-regulation remain unknown. Here, we demonstrate that the KNL1 N terminus is essential for Aurora B activity at kinetochores. This region of KNL1 is also required for Bub1 kinase activity at kinetochores, suggesting that KNL1 promotes Aurora B activity through Bub1-mediated Aurora B targeting. However, ectopic targeting of Aurora B to kinetochores does not fully rescue Aurora B activity in KNL1-depleted cells, suggesting KNL1 influences Aurora B activity through an additional pathway. Our findings establish KNL1 as a requirement for Aurora B activity at kinetochores and for wild-type kinetochore–MT attachment dynamics.
Figures




References
-
- Bolanos-Garcia V.M., Lischetti T., Matak-Vinković D., Cota E., Simpson P.J., Chirgadze D.Y., Spring D.R., Robinson C.V., Nilsson J., Blundell T.L. 2011. Structure of a Blinkin-BUBR1 complex reveals an interaction crucial for kinetochore-mitotic checkpoint regulation via an unanticipated binding site. Structure. 19:1691–1700 10.1016/j.str.2011.09.017 - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials
Miscellaneous

