Jab1 regulates Schwann cell proliferation and axonal sorting through p27
- PMID: 24344238
- PMCID: PMC3892969
- DOI: 10.1084/jem.20130720
Jab1 regulates Schwann cell proliferation and axonal sorting through p27
Abstract
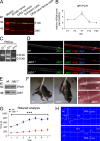
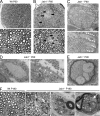
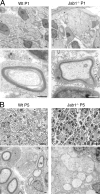
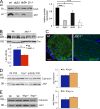
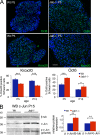
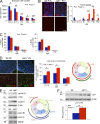
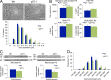
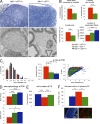
Axonal sorting is a crucial event in nerve formation and requires proper Schwann cell proliferation, differentiation, and contact with axons. Any defect in axonal sorting results in dysmyelinating peripheral neuropathies. Evidence from mouse models shows that axonal sorting is regulated by laminin211- and, possibly, neuregulin 1 (Nrg1)-derived signals. However, how these signals are integrated in Schwann cells is largely unknown. We now report that the nuclear Jun activation domain-binding protein 1 (Jab1) may transduce laminin211 signals to regulate Schwann cell number and differentiation during axonal sorting. Mice with inactivation of Jab1 in Schwann cells develop a dysmyelinating neuropathy with axonal sorting defects. Loss of Jab1 increases p27 levels in Schwann cells, which causes defective cell cycle progression and aberrant differentiation. Genetic down-regulation of p27 levels in Jab1-null mice restores Schwann cell number, differentiation, and axonal sorting and rescues the dysmyelinating neuropathy. Thus, Jab1 constitutes a regulatory molecule that integrates laminin211 signals in Schwann cells to govern cell cycle, cell number, and differentiation. Finally, Jab1 may constitute a key molecule in the pathogenesis of dysmyelinating neuropathies.
Figures
References
-
- Benninger Y., Thurnherr T., Pereira J.A., Krause S., Wu X., Chrostek-Grashoff A., Herzog D., Nave K.A., Franklin R.J., Meijer D., et al. 2007. Essential and distinct roles for cdc42 and rac1 in the regulation of Schwann cell biology during peripheral nervous system development. J. Cell Biol. 177:1051–1061 10.1083/jcb.200610108 - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials
Miscellaneous

