Initial stages of calcium uptake and mineral deposition in sea urchin embryos
- PMID: 24344263
- PMCID: PMC3890786
- DOI: 10.1073/pnas.1312833110
Initial stages of calcium uptake and mineral deposition in sea urchin embryos
Abstract
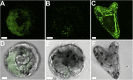
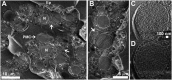
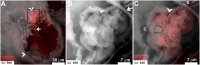
Sea urchin larvae have an endoskeleton consisting of two calcitic spicules. We reconstructed various stages of the formation pathway of calcium carbonate from calcium ions in sea water to mineral deposition and integration into the forming spicules. Monitoring calcium uptake with the fluorescent dye calcein shows that calcium ions first penetrate the embryo and later are deposited intracellularly. Surprisingly, calcium carbonate deposits are distributed widely all over the embryo, including in the primary mesenchyme cells and in the surface epithelial cells. Using cryo-SEM, we show that the intracellular calcium carbonate deposits are contained in vesicles of diameter 0.5-1.5 μm. Using the newly developed airSEM, which allows direct correlation between fluorescence and energy dispersive spectroscopy, we confirmed the presence of solid calcium carbonate in the vesicles. This mineral phase appears as aggregates of 20-30-nm nanospheres, consistent with amorphous calcium carbonate. The aggregates finally are introduced into the spicule compartment, where they integrate into the growing spicule.
Keywords: biomineralization; intracellular mineral deposition; mineralization pathway; sea urchin embryonic spicule; transient precursor mineral phase.
Conflict of interest statement
The authors declare no conflict of interest.
Figures



References
-
- Lowenstam HA, Weiner S. Transformation of amorphous calcium phosphate to crystalline dahillite in the radular teeth of chitons. Science. 1985;227(4682):51–53. - PubMed
-
- Addadi L, Raz S, Weiner S. Taking advantage of disorder: Amorphous calcium carbonate and its roles in biomineralization. Adv Mater. 2003;15(12):959–970.
-
- Nakano E, Okazaki K, Iwamatsu T. Accumulation of radioactive calcium in larvae of the sea urchin Pseudocentrotus depressus. Biol Bull. 1963;125(1):125–132.
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

