Rewiring yeast sugar transporter preference through modifying a conserved protein motif
- PMID: 24344268
- PMCID: PMC3890853
- DOI: 10.1073/pnas.1311970111
Rewiring yeast sugar transporter preference through modifying a conserved protein motif
Abstract
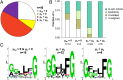
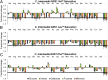
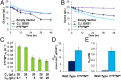
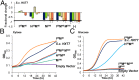
Utilization of exogenous sugars found in lignocellulosic biomass hydrolysates, such as xylose, must be improved before yeast can serve as an efficient biofuel and biochemical production platform. In particular, the first step in this process, the molecular transport of xylose into the cell, can serve as a significant flux bottleneck and is highly inhibited by other sugars. Here we demonstrate that sugar transport preference and kinetics can be rewired through the programming of a sequence motif of the general form G-G/F-XXX-G found in the first transmembrane span. By evaluating 46 different heterologously expressed transporters, we find that this motif is conserved among functional transporters and highly enriched in transporters that confer growth on xylose. Through saturation mutagenesis and subsequent rational mutagenesis, four transporter mutants unable to confer growth on glucose but able to sustain growth on xylose were engineered. Specifically, Candida intermedia gxs1 Phe(38)Ile(39)Met(40), Scheffersomyces stipitis rgt2 Phe(38) and Met(40), and Saccharomyces cerevisiae hxt7 Ile(39)Met(40)Met(340) all exhibit this phenotype. In these cases, primary hexose transporters were rewired into xylose transporters. These xylose transporters nevertheless remained inhibited by glucose. Furthermore, in the course of identifying this motif, novel wild-type transporters with superior monosaccharide growth profiles were discovered, namely S. stipitis RGT2 and Debaryomyces hansenii 2D01474. These findings build toward the engineering of efficient pentose utilization in yeast and provide a blueprint for reprogramming transporter properties.
Keywords: metabolic engineering; protein engineering; transporter engineering; xylose metabolism.
Conflict of interest statement
The authors declare no conflict of interest.
Figures



References
-
- Gárdonyi M, Jeppsson M, Lidén G, Gorwa-Grauslund MF, Hahn-Hägerdal B. Control of xylose consumption by xylose transport in recombinant Saccharomyces cerevisiae. Biotechnol Bioeng. 2003;82(7):818–824. - PubMed
-
- Wahlbom CF, Cordero Otero RR, van Zyl WH, Hahn-Hägerdal B, Jönsson LJ. Molecular analysis of a Saccharomyces cerevisiae mutant with improved ability to utilize xylose shows enhanced expression of proteins involved in transport, initial xylose metabolism, and the pentose phosphate pathway. Appl Environ Microbiol. 2003;69(2):740–746. - PMC - PubMed
-
- Bengtsson O, et al. Identification of common traits in improved xylose-growing Saccharomyces cerevisiae for inverse metabolic engineering. Yeast. 2008;25(11):835–847. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Miscellaneous

