Temporal course of changes in gene expression suggests a cytokine-related mechanism for long-term hippocampal alteration after controlled cortical impact
- PMID: 24344922
- PMCID: PMC3961781
- DOI: 10.1089/neu.2013.3029
Temporal course of changes in gene expression suggests a cytokine-related mechanism for long-term hippocampal alteration after controlled cortical impact
Abstract
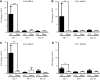
Mild traumatic brain injury (mTBI) often has long-term effects on cognitive function and social behavior. Altered gene expression may be predictive of long-term psychological effects of mTBI, even when acute clinical effects are minimal or transient. Controlled cortical impact (CCI), which causes concussive, but nonpenetrant, trauma to underlying (non-cortical) brain, resulting in persistent changes in hippocampal synaptic function, was used as a model of mTBI. The hippocampal transcriptomes of sham-operated or injured male rats at 1, 7, and 30 days postinjury were examined using microarrays comprising a comprehensive set of expressed genes, subsequently confirmed by quantitative reverse-transcriptase polymerase chain reaction. Transcripts encoding the chemokines, chemokine (C-C motif) ligand (Ccl)2 and Ccl7, inflammatory mediators lipocalin-2 (Lcn2) and tissue inhibitor of metalloproteinase 1 (Timp1), immunocyte activators C-C chemokine receptor type 5 (Ccr5) and Fc fragment of IgG, low affinity IIb, receptor (CD32) (Fcgr2b), the major histocompatibility complex II immune response-related genes, Cd74 and RT1 class II, locus Da (RT1-Da), the complement component, C3, and the transcription factor, Kruppel-like factor 4 (Klf4), were identified as early (Ccl2, Ccl7, Lcn2, and Timp1), intermediate (Ccr5, Fcgr2b, Cd74, RT1-Da, and C3), and late (Klf4) markers for bilateral hippocampal response to CCI. Ccl2 and Ccl7 transcripts were up-regulated within 24 h after CCI, and their elevation subsided within 1 week of injury. Other transcriptional changes occurred later and were more stable, some persisting for at least 1 month, suggesting that short-term inflammatory responses trigger longer-term alteration in the expression of genes previously associated with injury, aging, and neuronal function in the brain. These transcriptional responses to mTBI may underlie long-term changes in excitatory and inhibitory neuronal imbalance in hippocampus, leading to long-term behavioral consequences of mTBI.
Figures



References
-
- Coronado V.G., Xu L., Basavaraju S.V., McGuire L.C., Wald M.M., Faul M.D., Guzman B.R., and Hemphill J.D. (2011). Surveillance for traumatic brain injury-related deaths—United States, 1997–2007. MMWR Surveill. Summ. 60, 1–32 - PubMed
-
- Harvey A.G., and Bryant R.A. (2000). Two-year prospective evaluation of the relationship between acute stress disorder and posttraumatic stress disorder following mild traumatic brain injury. Am. J. Psychiatry 157, 626–628 - PubMed
-
- Hoge C.W., McGurk D., Thomas J.L., Cox A.L., Engel C.C., and Castro C.A. (2008). Mild traumatic brain injury in U.S. Soldiers returning from Iraq. N. Engl. J. Med. 358, 453–463 - PubMed
-
- Chavko M., Watanabe T., Adeeb S., Lankasky J., Ahlers S.T., and McCarron R.M. (2011). Relationship between orientation to a blast and pressure wave propagation inside the rat brain. J. Neurosci. Methods 195, 61–66 - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials
Miscellaneous

