Reorganization of retinotopic maps after occipital lobe infarction
- PMID: 24345177
- PMCID: PMC4435840
- DOI: 10.1162/jocn_a_00538
Reorganization of retinotopic maps after occipital lobe infarction
Abstract
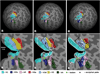
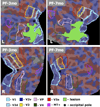
We studied patient JS, who had a right occipital infarct that encroached on visual areas V1, V2v, and VP. When tested psychophysically, he was very impaired at detecting the direction of motion in random dot displays where a variable proportion of dots moving in one direction (signal) were embedded in masking motion noise (noise dots). The impairment on this motion coherence task was especially marked when the display was presented to the upper left (affected) visual quadrant, contralateral to his lesion. However, with extensive training, by 11 months his threshold fell to the level of healthy participants. Training on the motion coherence task generalized to another motion task, the motion discontinuity task, on which he had to detect the presence of an edge that was defined by the difference in the direction of the coherently moving dots (signal) within the display. He was much better at this task at 8 than 3 months, and this improvement was associated with an increase in the activation of the human MT complex (hMT(+)) and in the kinetic occipital region as shown by repeated fMRI scans. We also used fMRI to perform retinotopic mapping at 3, 8, and 11 months after the infarct. We quantified the retinotopy and areal shifts by measuring the distances between the center of mass of functionally defined areas, computed in spherical surface-based coordinates. The functionally defined retinotopic areas V1, V2v, V2d, and VP were initially smaller in the lesioned right hemisphere, but they increased in size between 3 and 11 months. This change was not found in the normal, left hemisphere of the patient or in either hemispheres of the healthy control participants. We were interested in whether practice on the motion coherence task promoted the changes in the retinotopic maps. We compared the results for patient JS with those from another patient (PF) who had a comparable lesion but had not been given such practice. We found similar changes in the maps in the lesioned hemisphere of PF. However, PF was only scanned at 3 and 7 months, and the biggest shifts in patient JS were found between 8 and 11 months. Thus, it is important to carry out a prospective study with a trained and untrained group so as to determine whether the patterns of reorganization that we have observed can be further promoted by training.
Figures






References
-
- Azzopardi P, Cowey A. Motion discrimination in cortically blind patients. Brain; a Journal of Neurology. 2001;124:30–46. - PubMed
-
- Calford MB, Chino YM, Das A, et al. Neuroscience: rewiring the adult brain. Nature. 2005;438:E3. discussion E3–4. - PubMed
-
- Carmichael ST. Plasticity of cortical projections after stroke. Neuroscientist. 2003;9:64–75. - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

