The pathology of an autoimmune astrocytopathy: lessons learned from neuromyelitis optica
- PMID: 24345222
- PMCID: PMC3905574
- DOI: 10.1111/bpa.12099
The pathology of an autoimmune astrocytopathy: lessons learned from neuromyelitis optica
Abstract
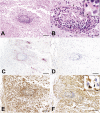
Neuromyelitis optica (NMO) is a disabling autoimmune astrocytopathy characterized by typically severe and recurrent attacks of optic neuritis and longitudinally extensive myelitis. Until recently, NMO was considered an acute aggressive variant of multiple sclerosis (MS), despite the fact that early studies postulated that NMO and MS may be two distinct diseases with a common clinical picture. With the discovery of a highly specific serum autoantibody (NMO-IgG), Lennon and colleagues provided the first unequivocal evidence distinguishing NMO from MS and other central nervous system (CNS) inflammatory demyelinating disorders. The target antigen of NMO-IgG was confirmed to be aquaporin-4 (AQP4), the most abundant water channel protein in the CNS, mainly expressed on astrocytic foot processes at the blood-brain barrier, subpial and subependymal regions. Pathological studies demonstrated that astrocytes were selectively targeted in NMO as evidenced by the extensive loss of immunoreactivities for the astrocytic proteins, AQP4 and glial fibrillary acidic protein (GFAP), as well as perivascular deposition of immunoglobulins and activation of complement even within lesions with a relative preservation of myelin. In support of these pathological findings, GFAP levels in the cerebrospinal fluid (CSF) during acute NMO exacerbations were found to be remarkably elevated in contrast to MS where CSF-GFAP levels did not substantially differ from controls. Additionally, recent experimental studies showed that AQP4 antibody is pathogenic, resulting in selective astrocyte destruction and dysfunction in vitro, ex vivo and in vivo. These findings strongly suggest that NMO is an autoimmune astrocytopathy where damage to astrocytes exceeds both myelin and neuronal damage. This chapter will review recent neuropathological studies that have provided novel insights into the pathogenic mechanisms, cellular targets, as well as the spectrum of tissue damage in NMO.
Keywords: aquaporin-4 (AQP4); astrocytopathy; neuromyelitis optica (NMO).
© 2013 International Society of Neuropathology.
Figures



References
-
- Afshari FT, Kwok JC, White L, Fawcett JW (2010) Schwann cell migration is integrin‐dependent and inhibited by astrocyte‐produced aggrecan. Glia 58:857–869. - PubMed
-
- Almekhlafi MA, Clark AW, Lucchinetti CF, Zhang Y, Power C, Bell RB (2011) Neuromyelitis optica with extensive active brain involvement: an autopsy study. Arch Neurol 68:508–512. - PubMed
-
- Apiwattanakul M, Popescu BF, Matiello M, Weinshenker BG, Lucchinetti CF, Lennon VA et al (2010) Intractable vomiting as the initial presentation of neuromyelitis optica. Ann Neurol 68:757–761. - PubMed
-
- Barnett MH, Prineas JW, Buckland ME, Parratt JD, Pollard JD (2012) Massive astrocyte destruction in neuromyelitis optica despite natalizumab therapy. Mult Scler 18:108–112. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

