Progesterone receptor assembly of a transcriptional complex along with activator protein 1, signal transducer and activator of transcription 3 and ErbB-2 governs breast cancer growth and predicts response to endocrine therapy
- PMID: 24345432
- PMCID: PMC3978912
- DOI: 10.1186/bcr3587
Progesterone receptor assembly of a transcriptional complex along with activator protein 1, signal transducer and activator of transcription 3 and ErbB-2 governs breast cancer growth and predicts response to endocrine therapy
Retraction in
-
Retraction Note: Progesterone receptor assembly of a transcriptional complex along with activator protein 1, signal transducer and activator of transcription 3 and ErbB-2 governs breast cancer growth and predicts response to endocrine therapy.Breast Cancer Res. 2023 Nov 2;25(1):133. doi: 10.1186/s13058-023-01735-z. Breast Cancer Res. 2023. PMID: 37919764 Free PMC article. No abstract available.
Abstract
Introduction: The role of the progesterone receptor (PR) in breast cancer remains a major clinical challenge. Although PR induces mammary tumor growth, its presence in breast tumors is a marker of good prognosis. We investigated coordinated PR rapid and nonclassical transcriptional effects governing breast cancer growth and endocrine therapy resistance.
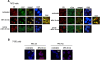
Methods: We used breast cancer cell lines expressing wild-type and mutant PRs, cells sensitive and resistant to endocrine therapy, a variety of molecular and cellular biology approaches, in vitro proliferation studies and preclinical models to explore PR regulation of cyclin D1 expression, tumor growth, and response to endocrine therapy. We investigated the clinical significance of activator protein 1 (AP-1) and PR interaction in a cohort of 99 PR-positive breast tumors by an immunofluorescence protocol we developed. The prognostic value of AP-1/PR nuclear colocalization in overall survival (OS) was evaluated using Kaplan-Meier method, and Cox model was used to explore said colocalization as an independent prognostic factor for OS.
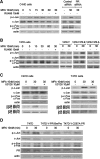
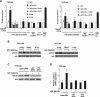
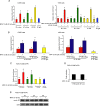
Results: We demonstrated that at the cyclin D1 promoter and through coordinated rapid and transcriptional effects, progestin induces the assembly of a transcriptional complex among AP-1, Stat3, PR, and ErbB-2 which functions as an enhanceosome to drive breast cancer growth. Our studies in a cohort of human breast tumors identified PR and AP-1 nuclear interaction as a marker of good prognosis and better OS in patients treated with tamoxifen (Tam), an anti-estrogen receptor therapy. Rationale for this finding was provided by our demonstration that Tam inhibits rapid and genomic PR effects, rendering breast cancer cells sensitive to its antiproliferative effects.
Conclusions: We here provided novel insight into the paradox of PR action as well as new tools to identify the subgroup of ER+/PR + patients unlikely to respond to ER-targeted therapies.
Figures
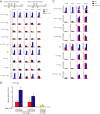


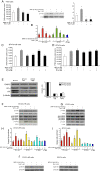
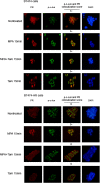
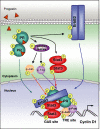
References
-
- Boonyaratanakornkit V, Scott MP, Ribon V, Sherman L, Anderson SM, Maller JL, Miller WT, Edwards DP. Progesterone receptor contains a proline-rich motif that directly interacts with SH3 domains and activates c-Src family tyrosine kinases. Mol Cell. 2001;15:269–280. doi: 10.1016/S1097-2765(01)00304-5. - DOI - PubMed
-
- Carnevale RP, Proietti CJ, Salatino M, Urtreger A, Peluffo G, Edwards DP, Boonyaratanakornkit V, Charreau EH, Bal de Kier JE, Schillaci R, Elizalde PV. Progestin effects on breast cancer cell proliferation, proteases activation, and in vivo development of metastatic phenotype all depend on progesterone receptor capacity to activate cytoplasmic signaling pathways. Mol Endocrinol. 2007;15:1335–1358. doi: 10.1210/me.2006-0304. - DOI - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials
Miscellaneous

