Static magnetic fields modulate X-ray-induced DNA damage in human glioblastoma primary cells
- PMID: 24345558
- PMCID: PMC3951070
- DOI: 10.1093/jrr/rrt107
Static magnetic fields modulate X-ray-induced DNA damage in human glioblastoma primary cells
Abstract
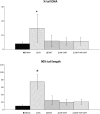
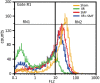
Although static magnetic fields (SMFs) are used extensively in the occupational and medical fields, few comprehensive studies have investigated their possible genotoxic effect and the findings are controversial. With the advent of magnetic resonance imaging-guided radiation therapy, the potential effects of SMFs on ionizing radiation (IR) have become increasingly important. In this study we focused on the genotoxic effect of 80 mT SMFs, both alone and in combination with (i.e. preceding or following) X-ray (XR) irradiation, on primary glioblastoma cells in culture. The cells were exposed to: (i) SMFs alone; (ii) XRs alone; (iii) XR, with SMFs applied during recovery; (iv) SMFs both before and after XR irradiation. XR-induced DNA damage was analyzed by Single Cell Gel Electrophoresis assay (comet assay) using statistical tools designed to assess the tail DNA (TD) and tail length (TL) as indicators of DNA fragmentation. Mitochondrial membrane potential, known to be affected by IR, was assessed using the JC-1 mitochondrial probe. Our results showed that exposure of cells to 5 Gy of XR irradiation alone led to extensive DNA damage, which was significantly reduced by post-irradiation exposure to SMFs. The XR-induced loss of mitochondrial membrane potential was to a large extent averted by exposure to SMFs. These data suggest that SMFs modulate DNA damage and/or damage repair, possibly through a mechanism that affects mitochondria.
Keywords: DNA fragmentation; comet assay; glioblastoma; ionizing radiation; mitochondrial membrane potential; static magnetic field.
Figures



References
-
- Schiffer IB, Schreiber WG, Graf R, et al. No influence of magnetic fields on cell cycle progression using conditions relevant for patients during MRI. Bioelectromagnetics. 2003;24:241–50. - PubMed
-
- Teodori L, Albertini MC, Uguccioni F, et al. Static magnetic fields affect cell size, shape, orientation, and membrane surface of human glioblastoma cells, as demonstrated by electron, optic, and atomic force microscopy. Cytometry A. 2006;69:75–85. - PubMed
-
- Coletti D, Teodori L, Albertini MC, et al. Static magnetic fields enhance skeletal muscle differentiation in vitro by improving myoblast alignment. Cytometry A. 2007;71:846–56. - PubMed
-
- Tenuzzo B, Chionna A, Panzarini E, et al. Biological effects of 6 mT static magnetic fields: a comparative study in different cell types. Bioelectromagnetics. 2006;27:560–77. - PubMed
-
- Chekhun VF, Lozovskaya YV, Lukyanova NY, et al. Assessment of the cyto- and genotoxic effects of a nanoferromagnetic and a static magnetic field in vivo. Cytol Genet. 2013;47:179–87.
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

