Influence of Polyethylene Glycol Lipid Desorption Rates on Pharmacokinetics and Pharmacodynamics of siRNA Lipid Nanoparticles
- PMID: 24345865
- PMCID: PMC3894582
- DOI: 10.1038/mtna.2013.66
Influence of Polyethylene Glycol Lipid Desorption Rates on Pharmacokinetics and Pharmacodynamics of siRNA Lipid Nanoparticles
Abstract
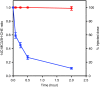
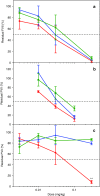
Lipid nanoparticles (LNPs) encapsulating short interfering RNAs that target hepatic genes are advancing through clinical trials, and early results indicate the excellent gene silencing observed in rodents and nonhuman primates also translates to humans. This success has motivated research to identify ways to further advance this delivery platform. Here, we characterize the polyethylene glycol lipid (PEG-lipid) components, which are required to control the self-assembly process during formation of lipid particles, but can negatively affect delivery to hepatocytes and hepatic gene silencing in vivo. The rate of transfer from LNPs to plasma lipoproteins in vivo is measured for three PEG-lipids with dialkyl chains 14, 16, and 18 carbons long. We show that 1.5 mol % PEG-lipid represents a threshold concentration at which the chain length exerts a minimal effect on hepatic gene silencing but can still modify LNPs pharmacokinetics and biodistribution. Increasing the concentration to 2.5 and 3.5 mol % substantially compromises hepatocyte gene knockdown for PEG-lipids with distearyl (C18) chains but has little impact for shorter dimyristyl (C14) chains. These data are discussed with respect to RNA delivery and the different rates at which the steric barrier disassociates from LNPs in vivo.Molecular Therapy-Nucleic Acids (2013) 2, e139; doi:10.1038/mtna.2013.66; published online 17 December 2013.
Figures



References
-
- Coelho T, Adams D, Silva A, Lozeron P, Hawkins PN, Mant T, et al. Safety and efficacy of RNAi therapy for transthyretin amyloidosis. N Engl J Med. 2013;369:819–829. - PubMed
-
- Fitzgerald K, Frank-Kamenetsky M, Shulga-Morskaya S, Liebow A, Bettencourt BR, Sutherland JE, et al. Effect of an RNA interference drug on the synthesis of proprotein convertase subtilisin/kexin type 9 (PCSK9) and the concentration of serum LDL cholesterol in healthy volunteers: a randomised, single-blind, placebo-controlled, phase 1 trial. Lancet. 2013. - PMC - PubMed
-
- Zimmermann TS, Lee AC, Akinc A, Bramlage B, Bumcrot D, Fedoruk MN, et al. RNAi-mediated gene silencing in non-human primates. Nature. 2006;441:111–114. - PubMed
LinkOut - more resources
Full Text Sources
Other Literature Sources

