The Wnt/planar cell polarity signaling pathway contributes to the integrity of tight junctions in brain endothelial cells
- PMID: 24346691
- PMCID: PMC3948118
- DOI: 10.1038/jcbfm.2013.213
The Wnt/planar cell polarity signaling pathway contributes to the integrity of tight junctions in brain endothelial cells
Abstract
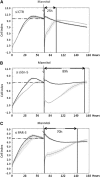
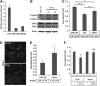
Wnt morphogens released by neural precursor cells were recently reported to control blood-brain barrier (BBB) formation during development. Indeed, in mouse brain endothelial cells, activation of the Wnt/β-catenin signaling pathway, also known as the canonical Wnt pathway, was shown to stabilize endothelial tight junctions (TJs) through transcriptional regulation of the expression of TJ proteins. Because Wnt proteins activate several distinct β-catenin-dependent and independent signaling pathways, this study was designed to assess whether the noncanonical Wnt/Par/aPKC planar cell polarity (PCP) pathway might also control TJ integrity in brain endothelial cells. First we established, in the hCMEC/D3 human brain endothelial cell line, that the Par/aPKC PCP complex colocalizes with TJs and controls apicobasal polarization. Second, using an siRNA approach, we showed that the Par/aPKC PCP complex regulates TJ stability and reassembling after osmotic shock. Finally, we provided evidence that Wnt5a signals in hCMEC/D3 cells through activation of the Par/aPKC PCP complex, independently of the Wnt canonical β-catenin-dependent pathway and significantly contributes to TJ integrity and endothelial apicobasal polarity. In conclusion, this study suggests that the Wnt/Par/aPKC PCP pathway, in addition to the Wnt/β-catenin canonical pathway, is a key regulator of the BBB.
Figures




References
-
- Abbott NJ, Patabendige AA, Dolman DE, Yusof SR, Begley DJ. Structure and function of the blood-brain barrier. Neurobiol Dis. 2010;37:13–25. - PubMed
-
- Tsukita S, Furuse M, Itoh M. Multifunctional strands in tight junctions. Nat Rev Mol Cell Biol. 2001;2:285–293. - PubMed
-
- Dragsten PR, Blumenthal R, Handler JS. Membrane asymmetry in epithelia: is the tight junction a barrier to diffusion in the plasma membrane. Nature. 1981;294:718–722. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources

