Optimized breast MRI functional tumor volume as a biomarker of recurrence-free survival following neoadjuvant chemotherapy
- PMID: 24347097
- PMCID: PMC4507716
- DOI: 10.1002/jmri.24351
Optimized breast MRI functional tumor volume as a biomarker of recurrence-free survival following neoadjuvant chemotherapy
Abstract
Purpose: To evaluate optimal contrast kinetics thresholds for measuring functional tumor volume (FTV) by breast magnetic resonance imaging (MRI) for assessment of recurrence-free survival (RFS).
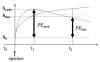
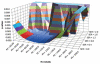
Materials and methods: In this Institutional Review Board (IRB)-approved retrospective study of 64 patients (ages 29-72, median age of 48.6) undergoing neoadjuvant chemotherapy (NACT) for breast cancer, all patients underwent pre-MRI1 and postchemotherapy MRI4 of the breast. Tumor was defined as voxels meeting thresholds for early percent enhancement (PEthresh) and early-to-late signal enhancement ratio (SERthresh); and FTV (PEthresh, SERthresh) by summing all voxels meeting threshold criteria and minimum connectivity requirements. Ranges of PEthresh from 50% to 220% and SERthresh from 0.0 to 2.0 were evaluated. A Cox proportional hazard model determined associations between change in FTV over treatment and RFS at different PE and SER thresholds.
Results: The plot of hazard ratios for change in FTV from MRI1 to MRI4 showed a broad peak with the maximum hazard ratio and highest significance occurring at PE threshold of 70% and SER threshold of 1.0 (hazard ratio = 8.71, 95% confidence interval 2.86-25.5, P < 0.00015), indicating optimal model fit.
Conclusion: Enhancement thresholds affect the ability of MRI tumor volume to predict RFS. The value is robust over a wide range of thresholds, supporting the use of FTV as a biomarker.
Keywords: MRI breast functional tumor volume; SER/PE thresholds; neoadjuvant chemotherapy.
© 2013 Wiley Periodicals, Inc.
Figures



References
-
- Hortobagyi GN. Progress in systemic chemotherapy of primary breast cancer: an overview. J Natl Cancer Inst Monogr. 2001:72–79. - PubMed
-
- Wolmark N, Wang J, Mamounas E, Bryant J, Fisher B. Preoperative chemotherapy in patients with operable breast cancer: nine-year results from National Surgical Adjuvant Breast and Bowel Project B-18. J Natl Cancer Inst Monogr. 2001;30:96–102. - PubMed
-
- Kuerer HM, Newman LA, Smith TL, et al. Clinical course of breast cancer patients with complete pathologic primary tumor and axillary lymph node response to doxorubicin-based neoadjuvant chemotherapy. J Clin Oncol. 1999;17:460–469. - PubMed
-
- Fisher B, Bryant J, Wolmark N, et al. Effect of preoperative chemotherapy on the outcome of women with operable breast cancer. J Clin Oncol. 1998;16:2672–2685. - PubMed
-
- Bonadonna G, Valagussa P, Brambilla C, et al. Primary chemotherapy in operable breast cancer: eight-year experience at the Milan Cancer Institute. J Clin Oncol. 1998;16:93–100. - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

