Spontaneous slow replication fork progression elicits mitosis alterations in homologous recombination-deficient mammalian cells
- PMID: 24347643
- PMCID: PMC3896206
- DOI: 10.1073/pnas.1311520111
Spontaneous slow replication fork progression elicits mitosis alterations in homologous recombination-deficient mammalian cells
Abstract
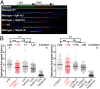
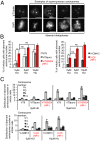
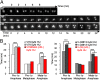
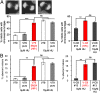
Homologous recombination deficient (HR(-)) mammalian cells spontaneously display reduced replication fork (RF) movement and mitotic extra centrosomes. We show here that these cells present a complex mitotic phenotype, including prolonged metaphase arrest, anaphase bridges, and multipolar segregations. We then asked whether the replication and the mitotic phenotypes are interdependent. First, we determined low doses of hydroxyurea that did not affect the cell cycle distribution or activate CHK1 phosphorylation but did slow the replication fork movement of wild-type cells to the same level than in HR(-) cells. Remarkably, these low hydroxyurea doses generated the same mitotic defects (and to the same extent) in wild-type cells as observed in unchallenged HR(-) cells. Reciprocally, supplying nucleotide precursors to HR(-) cells suppressed both their replication deceleration and mitotic extra centrosome phenotypes. Therefore, subtle replication stress that escapes to surveillance pathways and, thus, fails to prevent cells from entering mitosis alters metaphase progression and centrosome number, resulting in multipolar mitosis. Importantly, multipolar mitosis results in global unbalanced chromosome segregation involving the whole genome, even fully replicated chromosomes. These data highlight the cross-talk between chromosome replication and segregation, and the importance of HR at the interface of these two processes for protection against general genome instability.
Conflict of interest statement
The authors declare no conflict of interest.
Figures

References
-
- Bartkova J, et al. DNA damage response as a candidate anti-cancer barrier in early human tumorigenesis. Nature. 2005;434(7035):864–870. - PubMed
-
- Deng G, Lu Y, Zlotnikov G, Thor AD, Smith HS. Loss of heterozygosity in normal tissue adjacent to breast carcinomas. Science. 1996;274(5295):2057–2059. - PubMed
-
- Gorgoulis VG, et al. Activation of the DNA damage checkpoint and genomic instability in human precancerous lesions. Nature. 2005;434(7035):907–913. - PubMed
-
- Krämer A. Centrosome aberrations—hen or egg in cancer initiation and progression? Leukemia. 2005;19(7):1142–1144. - PubMed
-
- Lakhani SR, et al. Genetic alterations in ‘normal’ luminal and myoepithelial cells of the breast. J Pathol. 1999;189(4):496–503. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

