β-Pyrazino-fused tetrarylporphyrins
- PMID: 24347747
- PMCID: PMC3863362
- DOI: 10.1016/j.dyepig.2013.04.024
β-Pyrazino-fused tetrarylporphyrins
Abstract
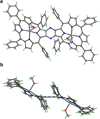
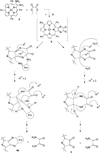
A novel method for the preparation of β-fused porphyrin dyads was developed that exploits a one-pot reaction of 2,3-diaminoporphyrins with diethyl oxalate. This approach provides good yields of the zinc β-fused dyad and the corresponding free-base, opening the way for preparation of several metal derivatives to permit modulation of optoelectronic characteristics for commercial applications.
Keywords: 2,3-Diaminoporphyrin; DSSC; Dietyl oxalate; Porphyrin dyad electrochemistry; α-dione porphyrin; β-fused-porphyrin.
Figures






References
-
- Barker CA, Zeng XS, Bettington S, Batsanov AS, Bryce MR, Beeby A. Porphyrin, phthalocyanine and porphyrazine derivatives with multifluorenyl substituents as efficient deep-red emitters. Chem Eur J. 2007;13:6710–6717. - PubMed
- Pandey R, Zheng G. Porphyrins as photosensitizers in photodynamic therapy. In: Kadish KM, Smith KM, Guilard R, editors. The porphyrin handbook. vol. 6. San Diego: Academic Press; 2000. pp. 157–225.
- Lebedev AY, Filatov MA, Cheprakov AV, Vinagradov SA. Effects of structural deformations on optical properties of tetrabenzoporphyrins: free-bases and Pd complexes. J Phys Chem A. 2008;112:7723–7733. - PMC - PubMed
-
- Martinez-Diaz MV, de la Torre G, Torres T. Lighting porphyrins and phthalocyanines for molecular photovoltaics. Chem Commun. 2010;46:7090–7108. - PubMed
-
- Yella A, Lee HW, Tsao HN, Yi C, Chandiran AK, Nazeeruddin MK, et al. Porphyrin-sensitized solar cells with cobalt (II/III)-based redox electrolyte exceed 12 percent efficiency. Science. 2011;334:629–634. - PubMed
-
- Mai C-L, Huang W-K, Lu H-P, Lee C-W, Chiu C-L, Liang Y-R, et al. Synthesis and characterization of diporphyrin sensitizers for dye-sensitized solar cells. Chem Commun. 2010;46:809–811. - PubMed
-
- Sessler JL, Gebauer A, Vogel E. Expanded porphyrins. In: Kadish KM, Smith KM, Guilard R, editors. The porphyrin handbook. vol. 2. San Diego: Academic Press; 2000. pp. 55–121.
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
