The risk of bleeding with duloxetine treatment in patients who use nonsteroidal anti-inflammatory drugs (NSAIDs): analysis of placebo-controlled trials and post-marketing adverse event reports
- PMID: 24348072
- PMCID: PMC3849082
- DOI: 10.2147/DHPS.S45445
The risk of bleeding with duloxetine treatment in patients who use nonsteroidal anti-inflammatory drugs (NSAIDs): analysis of placebo-controlled trials and post-marketing adverse event reports
Abstract
Purpose: To assess the safety of duloxetine with regards to bleeding-related events in patients who concomitantly did, versus did not, use nonsteroidal anti-inflammatory drugs (NSAIDs), including aspirin.
Methods: Safety data from all placebo-controlled trials of duloxetine conducted between December 1993 and December 2010, and post-marketing reports from duloxetine-treated patients in the US Food and Drug Administration Adverse Event Reporting System (FAERS), were searched for bleeding-related treatment-emergent adverse events (TEAEs). The percentage of patients with bleeding-related TEAEs was summarized and compared between treatment groups in all the placebo-controlled studies. Differences between NSAID user and non-user subgroups from clinical trial data were analyzed by a logistic regression model that included therapy, NSAID use, and therapy-by-NSAID subgroup interaction. In addition, to determine if higher duloxetine doses are associated with an increased incidence of bleeding-related TEAEs, and whether the use of concomitant NSAIDs might influence the dose effect if one exists, placebo-controlled clinical trials with duloxetine fixed doses of 60 mg, 120 mg, and placebo were analyzed. Also, the incidence of bleeding-related TEAEs reported for duloxetine alone was compared with the incidence in patients treated with duloxetine and concomitant NSAIDs. Finally, the number of bleeding-related cases reported for duloxetine in the FAERS database was compared with the numbers reported for all other drugs.
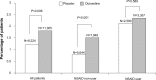
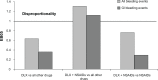
Results: Across duloxetine clinical trials, there was a significantly greater incidence of bleeding-related TEAEs in duloxetine- versus placebo-treated patients overall and also in those patients who did not take concomitant NSAIDS, but no significant difference was seen among those patients who did take concomitant NSAIDS. There was no significant difference in the incidence of bleeding-related TEAEs in the subset of patients treated with duloxetine 120 mg once daily versus those treated with 60 mg once daily regardless of concomitant NSAID use. The combination of duloxetine and NSAIDs was associated with a statistically significantly higher incidence of bleeding-related TEAEs compared with duloxetine alone. A similarly higher incidence of bleeding-related TEAEs was seen in patients treated with placebo and concomitant NSAIDs compared with placebo alone. Bleeding-related TEAEs reported in the FAERS database were disproportionally more frequent for duloxetine taken with NSAIDs compared with the full FAERS background, but there was no difference in the reporting of bleeding-related TEAEs when the cases reported for duloxetine taken with NSAIDs were compared against the cases reported for NSAIDs alone.
Conclusion: Concomitant use of NSAIDs was associated with a higher incidence of bleeding-related TEAEs in clinical trials regardless of whether patients were taking duloxetine or placebo; bleeding-related TEAEs did not appear to increase along with duloxetine dose regardless of NSAID use. In spontaneously reported post-marketing data, the combination of duloxetine and NSAID use was not associated with an increased reporting of bleeding-related events when compared to NSAID use alone.
Keywords: NSAID; antidepressant; aspirin; gastrointestinal bleeding.
Figures

Similar articles
-
Observational study of upper gastrointestinal tract bleeding events in patients taking duloxetine and nonsteroidal anti-inflammatory drugs: a case-control analysis.Drug Healthc Patient Saf. 2014 Oct 29;6:167-74. doi: 10.2147/DHPS.S66835. eCollection 2014. Drug Healthc Patient Saf. 2014. PMID: 25382984 Free PMC article.
-
Efficacy and Safety of Duloxetine in Patients with Chronic Low Back Pain Who Used versus Did Not Use Concomitant Nonsteroidal Anti-Inflammatory Drugs or Acetaminophen: A Post Hoc Pooled Analysis of 2 Randomized, Placebo-Controlled Trials.Pain Res Treat. 2012;2012:296710. doi: 10.1155/2012/296710. Epub 2012 Mar 18. Pain Res Treat. 2012. PMID: 22550577 Free PMC article.
-
Efficacy of duloxetine by prior NSAID use in the treatment of chronic osteoarthritis knee pain: A post hoc subgroup analysis of a randomized, placebo-controlled, phase 3 study in Japan.J Orthop Sci. 2018 Nov;23(6):1019-1026. doi: 10.1016/j.jos.2018.07.008. Epub 2018 Aug 17. J Orthop Sci. 2018. PMID: 30126675 Clinical Trial.
-
Nonsteroidal anti-inflammatory drugs: add an anti-ulcer drug for patients at high risk only. Always limit the dose and duration of treatment with NSAIDs.Prescrire Int. 2011 Sep;20(119):216-9. Prescrire Int. 2011. PMID: 21954519 Review.
-
Systematic reviews of the clinical effectiveness and cost-effectiveness of proton pump inhibitors in acute upper gastrointestinal bleeding.Health Technol Assess. 2007 Dec;11(51):iii-iv, 1-164. doi: 10.3310/hta11510. Health Technol Assess. 2007. PMID: 18021578 Review.
Cited by
-
Exacerbation of Intracranial and Gastrointestinal Bleeding in Patients Above 50 Years of Age Co-treated With Antidepressants and Anticoagulants/Platelet Inhibitors at a Lebanese University Hospital.Cureus. 2025 May 13;17(5):e84033. doi: 10.7759/cureus.84033. eCollection 2025 May. Cureus. 2025. PMID: 40510108 Free PMC article.
-
Evaluating the Quality of Reports About Randomized Controlled Trials of Acupuncture for Low Back Pain.J Pain Res. 2021 Apr 21;14:1141-1151. doi: 10.2147/JPR.S308006. eCollection 2021. J Pain Res. 2021. PMID: 33911896 Free PMC article. Review.
-
Ibrutinib-associated bleeding: pathogenesis, management and risk reduction strategies.J Thromb Haemost. 2017 May;15(5):835-847. doi: 10.1111/jth.13651. Epub 2017 Mar 27. J Thromb Haemost. 2017. PMID: 28182323 Free PMC article. Review.
-
The Prevalence of Selected Potential Drug-Drug Interactions of Analgesic Drugs and Possible Methods of Preventing Them: Lessons Learned From the Analysis of the Real-World National Database of 38 Million Citizens of Poland.Front Pharmacol. 2021 Jan 18;11:607852. doi: 10.3389/fphar.2020.607852. eCollection 2020. Front Pharmacol. 2021. PMID: 33536918 Free PMC article.
-
Observational study of upper gastrointestinal tract bleeding events in patients taking duloxetine and nonsteroidal anti-inflammatory drugs: a case-control analysis.Drug Healthc Patient Saf. 2014 Oct 29;6:167-74. doi: 10.2147/DHPS.S66835. eCollection 2014. Drug Healthc Patient Saf. 2014. PMID: 25382984 Free PMC article.
References
-
- Goldberg RJ. Selective serotonin reuptake inhibitors: infrequent medical adverse effects. Arch Fam Med. 1998;7(1):78–84. - PubMed
-
- Andrade C, Sandarsh S, Chethan KB, Nagesha KS. Serotonin reuptake inhibitor antidepressants and abnormal bleeding: a review for clinicians and a reconsideration of mechanisms. J Clin Psychiatry. 2010;71(12):1565–1575. - PubMed
-
- Salvo F, Fourrier-Réglat A, Bazin F, et al. Investigators of Safety of Non-Steroidal Anti-Inflammatory Drugs: SOS Project Cardiovascular and gastrointestinal safety of NSAIDs: a systematic review of meta-analyses of randomized clinical trials. Clin Pharmacol Ther. 2011;89(6):855–866. - PubMed
-
- Dalton SO, Johansen C, Mellemkjær L, Nørgård B, Sørensen HT, Olsen JH. Use of selective serotonin reuptake inhibitors and risk of upper gastrointestinal tract bleeding: a population-based cohort study. Arch Intern Med. 2003;163(1):59–64. - PubMed
LinkOut - more resources
Full Text Sources
Other Literature Sources

