PTEN phosphatase-independent maintenance of glandular morphology in a predictive colorectal cancer model system
- PMID: 24348097
- PMCID: PMC3858899
- DOI: 10.1593/neo.121516
PTEN phosphatase-independent maintenance of glandular morphology in a predictive colorectal cancer model system
Abstract
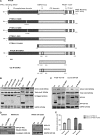
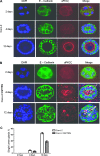
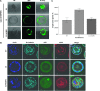
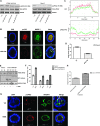
Organotypic models may provide mechanistic insight into colorectal cancer (CRC) morphology. Three-dimensional (3D) colorectal gland formation is regulated by phosphatase and tensin homologue deleted on chromosome 10 (PTEN) coupling of cell division cycle 42 (cdc42) to atypical protein kinase C (aPKC). This study investigated PTEN phosphatase-dependent and phosphatase-independent morphogenic functions in 3D models and assessed translational relevance in human studies. Isogenic PTEN-expressing or PTEN-deficient 3D colorectal cultures were used. In translational studies, apical aPKC activity readout was assessed against apical membrane (AM) orientation and gland morphology in 3D models and human CRC. We found that catalytically active or inactive PTEN constructs containing an intact C2 domain enhanced cdc42 activity, whereas mutants of the C2 domain calcium binding region 3 membrane-binding loop (M-CBR3) were ineffective. The isolated PTEN C2 domain (C2) accumulated in membrane fractions, but C2 M-CBR3 remained in cytosol. Transfection of C2 but not C2 M-CBR3 rescued defective AM orientation and 3D morphogenesis of PTEN-deficient Caco-2 cultures. The signal intensity of apical phospho-aPKC correlated with that of Na(+)/H(+) exchanger regulatory factor-1 (NHERF-1) in the 3D model. Apical NHERF-1 intensity thus provided readout of apical aPKC activity and associated with glandular morphology in the model system and human colon. Low apical NHERF-1 intensity in CRC associated with disruption of glandular architecture, high cancer grade, and metastatic dissemination. We conclude that the membrane-binding function of the catalytically inert PTEN C2 domain influences cdc42/aPKC-dependent AM dynamics and gland formation in a highly relevant 3D CRC morphogenesis model system.
Figures

Similar articles
-
Rescue of glandular dysmorphogenesis in PTEN-deficient colorectal cancer epithelium by PPARγ-targeted therapy.Oncogene. 2013 Mar 7;32(10):1305-15. doi: 10.1038/onc.2012.140. Epub 2012 Apr 30. Oncogene. 2013. PMID: 22543585 Free PMC article.
-
Vitamin D3 suppresses morphological evolution of the cribriform cancerous phenotype.Oncotarget. 2016 Aug 2;7(31):49042-49064. doi: 10.18632/oncotarget.8863. Oncotarget. 2016. PMID: 27119498 Free PMC article.
-
PTEN controls glandular morphogenesis through a juxtamembrane β-Arrestin1/ARHGAP21 scaffolding complex.Elife. 2017 Jul 27;6:e24578. doi: 10.7554/eLife.24578. Elife. 2017. PMID: 28749339 Free PMC article.
-
Phosphoinositides specify polarity during epithelial organ development.Cell. 2007 Jan 26;128(2):239-40. doi: 10.1016/j.cell.2007.01.010. Cell. 2007. PMID: 17254962 Review.
-
Acute regulation of the tumour suppressor phosphatase, PTEN, by anionic lipids and reactive oxygen species.Biochem Soc Trans. 2004 Apr;32(Pt 2):338-42. doi: 10.1042/bst0320338. Biochem Soc Trans. 2004. PMID: 15046604 Review.
Cited by
-
Protein kinase C zeta suppresses low- or high-grade colorectal cancer (CRC) phenotypes by interphase centrosome anchoring.J Pathol. 2018 Apr;244(4):445-459. doi: 10.1002/path.5035. Epub 2018 Mar 9. J Pathol. 2018. PMID: 29520890 Free PMC article.
-
Mechanistic Insights into Colorectal Cancer Phenomics from Fundamental and Organotypic Model Studies.Am J Pathol. 2018 Sep;188(9):1936-1948. doi: 10.1016/j.ajpath.2018.05.021. Epub 2018 Jul 18. Am J Pathol. 2018. PMID: 30028958 Free PMC article. Review.
-
Need for multi-scale systems to identify spindle orientation regulators relevant to tissue disorganization in solid cancers.Front Physiol. 2014 Jul 25;5:278. doi: 10.3389/fphys.2014.00278. eCollection 2014. Front Physiol. 2014. PMID: 25120491 Free PMC article. No abstract available.
-
Antisense oligonucleotides against microRNA-21 reduced the proliferation and migration of human colon carcinoma cells.Cancer Cell Int. 2015 Aug 1;15:77. doi: 10.1186/s12935-015-0228-7. eCollection 2015. Cancer Cell Int. 2015. PMID: 26236156 Free PMC article.
-
Preliminary Report: Asymmetric Crypt Fission in Biopsies from Patients With Ulcerative Colitis.In Vivo. 2020 Sep-Oct;34(5):2693-2695. doi: 10.21873/invivo.12089. In Vivo. 2020. PMID: 32871801 Free PMC article.
References
-
- Siegel R, Naishadham D, Jemal A. Cancer statistics, 2012. CA Cancer J Clin. 2012;62:10–29. - PubMed
-
- Compton CC, Fielding LP, Burgart LJ, Conley B, Cooper HS, Hamilton SR, Hammond ME, Henson DE, Hutter RV, Nagle RB, et al. Prognostic factors in colorectal cancer. College of American Pathologists Consensus Statement 1999. Arch Pathol Lab Med. 2000;124:979–994. - PubMed
-
- Härmä V, Knuuttila M, Virtanen J, Mirtti T, Kohonen P, Kovanen P, Happonen A, Kaewphan S, Ahonen I, Kallioniemi O, et al. Lysophosphatidic acid and sphingosine-1-phosphate promote morphogenesis and block invasion of prostate cancer cells in three-dimensional organotypic models. Oncogene. 2012;31:2075–2089. - PMC - PubMed
-
- Friedl P, Gilmour D. Collective cell migration in morphogenesis, regeneration and cancer. Nat Rev Mol Cell Biol. 2009;10:445–457. - PubMed
-
- Ueno H, Mochizuki H, Hashiguchi Y, Ishiguro M, Kajiwara Y, Sato T, Shimazaki H, Hase K, Talbot IC. Histological grading of colorectal cancer: a simple and objective method. Ann Surg. 2008;247:811–818. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials
Miscellaneous
