Cure kinetics of epoxy nanocomposites affected by MWCNTs functionalization: a review
- PMID: 24348181
- PMCID: PMC3853241
- DOI: 10.1155/2013/703708
Cure kinetics of epoxy nanocomposites affected by MWCNTs functionalization: a review
Abstract
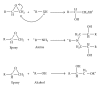
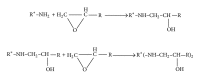
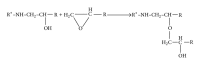
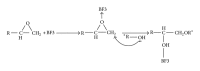
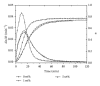
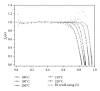
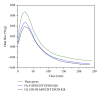
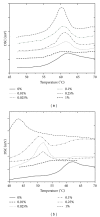
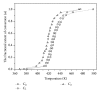
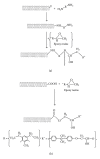
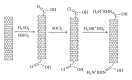
The current paper provides an overview to emphasize the role of functionalization of multiwalled carbon nanotubes (MWCNTs) in manipulating cure kinetics of epoxy nanocomposites, which itself determines ultimate properties of the resulting compound. In this regard, the most commonly used functionalization schemes, that is, carboxylation and amidation, are thoroughly surveyed to highlight the role of functionalized nanotubes in controlling the rate of autocatalytic and vitrification kinetics. The current literature elucidates that the mechanism of curing in epoxy/MWCNTs nanocomposites remains almost unaffected by the functionalization of carbon nanotubes. On the other hand, early stage facilitation of autocatalytic reactions in the presence of MWCNTs bearing amine groups has been addressed by several researchers. When carboxylated nanotubes were used to modify MWCNTs, the rate of such reactions diminished as a consequence of heterogeneous dispersion within the epoxy matrix. At later stages of curing, however, the prolonged vitrification was seen to be dominant. Thus, the type of functional groups covalently located on the surface of MWCNTs directly affects the degree of polymer-nanotube interaction followed by enhancement of curing reaction. Our survey demonstrated that most widespread efforts ever made to represent multifarious surface-treated MWCNTs have not been directed towards preparation of epoxy nanocomposites, but they could result in property synergism.
Figures




References
-
- Iijima S. Helical microtubules of graphitic carbon. Nature. 1991;354(6348):56–58.
-
- Ajayan PM, Stephan O, Colliex C, Trauth D. Aligned carbon nanotube arrays formed by cutting a polymer resin-nanotube composite. Science. 1994;265(5176):1212–1214. - PubMed
-
- Lee SH, Cho E, Jeon SH, Youn JR. Rheological and electrical properties of polypropylene composites containing functionalized multi-walled carbon nanotubes and compatibilizers. Carbon. 2007;45(14):2810–2822.
-
- Du F, Scogna RC, Zhou W, Brand S, Fischer JE, Winey KI. Nanotube networks in polymer nanocomposites: rheology and electrical conductivity. Macromolecules. 2004;37(24):9048–9055.
-
- Vega JF, Martínez-Salazar J, Trujillo M, et al. Rheology, processing, tensile properties, and crystallization of polyethylene/carbon nanotube nanocomposites. Macromolecules. 2009;42(13):4719–4727.
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources

