Dual expression profile of type VI secretion system immunity genes protects pandemic Vibrio cholerae
- PMID: 24348240
- PMCID: PMC3857813
- DOI: 10.1371/journal.ppat.1003752
Dual expression profile of type VI secretion system immunity genes protects pandemic Vibrio cholerae
Abstract
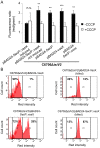
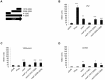
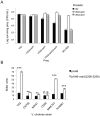
The Vibrio cholerae type VI secretion system (T6SS) assembles as a molecular syringe that injects toxic protein effectors into both eukaryotic and prokaryotic cells. We previously reported that the V. cholerae O37 serogroup strain V52 maintains a constitutively active T6SS to kill other Gram-negative bacteria while being immune to attack by kin bacteria. The pandemic O1 El Tor V. cholerae strain C6706 is T6SS-silent under laboratory conditions as it does not produce T6SS structural components and effectors, and fails to kill Escherichia coli prey. Yet, C6706 exhibits full resistance when approached by T6SS-active V52. These findings suggested that an active T6SS is not required for immunity against T6SS-mediated virulence. Here, we describe a dual expression profile of the T6SS immunity protein-encoding genes tsiV1, tsiV2, and tsiV3 that provides pandemic V. cholerae strains with T6SS immunity and allows T6SS-silent strains to maintain immunity against attacks by T6SS-active bacterial neighbors. The dual expression profile allows transcription of the three genes encoding immunity proteins independently of other T6SS proteins encoded within the same operon. One of these immunity proteins, TsiV2, protects against the T6SS effector VasX which is encoded immediately upstream of tsiV2. VasX is a secreted, lipid-binding protein that we previously characterized with respect to T6SS-mediated virulence towards the social amoeba Dictyostelium discoideum. Our data suggest the presence of an internal promoter in the open reading frame of vasX that drives expression of the downstream gene tsiV2. Furthermore, VasX is shown to act in conjunction with VasW, an accessory protein to VasX, to compromise the inner membrane of prokaryotic target cells. The dual regulatory profile of the T6SS immunity protein-encoding genes tsiV1, tsiV2, and tsiV3 permits V. cholerae to tightly control T6SS gene expression while maintaining immunity to T6SS activity.
Conflict of interest statement
The authors have declared that no competing interests exist.
Figures




Similar articles
-
Constitutive type VI secretion system expression gives Vibrio cholerae intra- and interspecific competitive advantages.PLoS One. 2012;7(10):e48320. doi: 10.1371/journal.pone.0048320. Epub 2012 Oct 26. PLoS One. 2012. PMID: 23110230 Free PMC article.
-
Vibrio cholerae requires the type VI secretion system virulence factor VasX to kill Dictyostelium discoideum.Infect Immun. 2011 Jul;79(7):2941-9. doi: 10.1128/IAI.01266-10. Epub 2011 May 9. Infect Immun. 2011. PMID: 21555399 Free PMC article.
-
Genetic analysis of anti-amoebae and anti-bacterial activities of the type VI secretion system in Vibrio cholerae.PLoS One. 2011;6(8):e23876. doi: 10.1371/journal.pone.0023876. Epub 2011 Aug 31. PLoS One. 2011. PMID: 21909372 Free PMC article.
-
T6SS intraspecific competition orchestrates Vibrio cholerae genotypic diversity.Int Microbiol. 2017 Sep;20(3):130-137. doi: 10.2436/20.1501.01.294. Int Microbiol. 2017. PMID: 29446804 Review.
-
Rules of Engagement: The Type VI Secretion System in Vibrio cholerae.Trends Microbiol. 2017 Apr;25(4):267-279. doi: 10.1016/j.tim.2016.12.003. Epub 2016 Dec 24. Trends Microbiol. 2017. PMID: 28027803 Free PMC article. Review.
Cited by
-
Pesticin-Like Effector VgrG3cp Targeting Peptidoglycan Delivered by the Type VI Secretion System Contributes to Vibrio cholerae Interbacterial Competition.Microbiol Spectr. 2023 Feb 14;11(1):e0426722. doi: 10.1128/spectrum.04267-22. Epub 2023 Jan 10. Microbiol Spectr. 2023. PMID: 36625646 Free PMC article.
-
Abiotic factors modulate interspecies competition mediated by the type VI secretion system effectors in Vibrio cholerae.ISME J. 2022 Jul;16(7):1765-1775. doi: 10.1038/s41396-022-01228-5. Epub 2022 Mar 30. ISME J. 2022. PMID: 35354946 Free PMC article.
-
Lytic transglycosylases RlpA and MltC assist in Vibrio cholerae daughter cell separation.Mol Microbiol. 2019 Oct;112(4):1100-1115. doi: 10.1111/mmi.14349. Epub 2019 Aug 8. Mol Microbiol. 2019. PMID: 31286580 Free PMC article.
-
Bile Salts Modulate the Mucin-Activated Type VI Secretion System of Pandemic Vibrio cholerae.PLoS Negl Trop Dis. 2015 Aug 28;9(8):e0004031. doi: 10.1371/journal.pntd.0004031. eCollection 2015. PLoS Negl Trop Dis. 2015. PMID: 26317760 Free PMC article.
-
A novel chaperone-effector-immunity system identified in uropathogenic Escherichia coli UMN026.PeerJ. 2024 May 20;12:e17336. doi: 10.7717/peerj.17336. eCollection 2024. PeerJ. 2024. PMID: 38784397 Free PMC article.
References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

