The type-specific neutralizing antibody response elicited by a dengue vaccine candidate is focused on two amino acids of the envelope protein
- PMID: 24348242
- PMCID: PMC3857832
- DOI: 10.1371/journal.ppat.1003761
The type-specific neutralizing antibody response elicited by a dengue vaccine candidate is focused on two amino acids of the envelope protein
Abstract
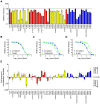
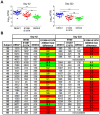
Dengue viruses are mosquito-borne flaviviruses that circulate in nature as four distinct serotypes (DENV1-4). These emerging pathogens are responsible for more than 100 million human infections annually. Severe clinical manifestations of disease are predominantly associated with a secondary infection by a heterotypic DENV serotype. The increased risk of severe disease in DENV-sensitized populations significantly complicates vaccine development, as a vaccine must simultaneously confer protection against all four DENV serotypes. Eliciting a protective tetravalent neutralizing antibody response is a major goal of ongoing vaccine development efforts. However, a recent large clinical trial of a candidate live-attenuated DENV vaccine revealed low protective efficacy despite eliciting a neutralizing antibody response, highlighting the need for a better understanding of the humoral immune response against dengue infection. In this study, we sought to identify epitopes recognized by serotype-specific neutralizing antibodies elicited by monovalent DENV1 vaccination. We constructed a panel of over 50 DENV1 structural gene variants containing substitutions at surface-accessible residues of the envelope (E) protein to match the corresponding DENV2 sequence. Amino acids that contribute to recognition by serotype-specific neutralizing antibodies were identified as DENV mutants with reduced sensitivity to neutralization by DENV1 immune sera, but not cross-reactive neutralizing antibodies elicited by DENV2 vaccination. We identified two mutations (E126K and E157K) that contribute significantly to type-specific recognition by polyclonal DENV1 immune sera. Longitudinal and cross-sectional analysis of sera from 24 participants of a phase I clinical study revealed a markedly reduced capacity to neutralize a E126K/E157K DENV1 variant. Sera from 77% of subjects recognized the E126K/E157K DENV1 variant and DENV2 equivalently (<3-fold difference). These data indicate the type-specific component of the DENV1 neutralizing antibody response to vaccination is strikingly focused on just two amino acids of the E protein. This study provides an important step towards deconvoluting the functional complexity of DENV serology following vaccination.
Conflict of interest statement
I have read the journal's policy and have the following conflicts. SSW is an inventor on US and Foreign issued patents for the DENV live attenuated vaccines studied within. This does not alter our adherence to all PLoS Pathogens policies on sharing data and materials.
Figures





References
-
- Halstead SB (2007) Dengue. Lancet 370: 1644–1652. - PubMed
-
- Guzman MG, Alvarez M, Halstead SB (2013) Secondary infection as a risk factor for dengue hemorrhagic fever/dengue shock syndrome: an historical perspective and role of antibody-dependent enhancement of infection. Arch Virol 158 ((7)): 1445–59. - PubMed
-
- Vaughn DW, Green S, Kalayanarooj S, Innis BL, Nimmannitya S, et al. (2000) Dengue viremia titer, antibody response pattern, and virus serotype correlate with disease severity. J Infect Dis 181: 2–9. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials

