Tmprss2 is essential for influenza H1N1 virus pathogenesis in mice
- PMID: 24348248
- PMCID: PMC3857797
- DOI: 10.1371/journal.ppat.1003774
Tmprss2 is essential for influenza H1N1 virus pathogenesis in mice
Erratum in
- PLoS Pathog. 2014 Sep;10(9):e1004435. Pöhlman, Stefan [corrected to Pöhlmann, Stefan]
Abstract
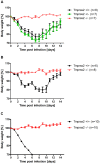
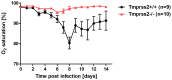
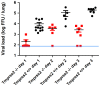
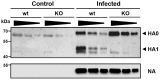
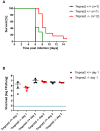
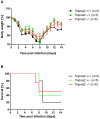
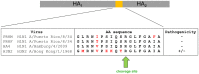
Annual influenza epidemics and occasional pandemics pose a severe threat to human health. Host cell factors required for viral spread but not for cellular survival are attractive targets for novel approaches to antiviral intervention. The cleavage activation of the influenza virus hemagglutinin (HA) by host cell proteases is essential for viral infectivity. However, it is unknown which proteases activate influenza viruses in mammals. Several candidates have been identified in cell culture studies, leading to the concept that influenza viruses can employ multiple enzymes to ensure their cleavage activation in the host. Here, we show that deletion of a single HA-activating protease gene, Tmprss2, in mice inhibits spread of mono-basic H1N1 influenza viruses, including the pandemic 2009 swine influenza virus. Lung pathology was strongly reduced and mutant mice were protected from weight loss, death and impairment of lung function. Also, after infection with mono-basic H3N2 influenza A virus body weight loss and survival was less severe in Tmprss2 mutant compared to wild type mice. As expected, Tmprss2-deficient mice were not protected from viral spread and pathology after infection with multi-basic H7N7 influenza A virus. In conclusion, these results identify TMPRSS2 as a host cell factor essential for viral spread and pathogenesis of mono-basic H1N1 and H3N2 influenza A viruses.
Conflict of interest statement
The authors have declared that no competing interests exist.
Figures


References
-
- Hurt AC, Chotpitayasunondh T, Cox NJ, Daniels R, Fry AM, et al. (2012) Antiviral resistance during the 2009 influenza A H1N1 pandemic: public health, laboratory, and clinical perspectives. Lancet Infect Dis 12: 240–248. - PubMed
-
- Klenk HD, Rott R, Orlich M, Blodorn J (1975) Activation of influenza A viruses by trypsin treatment. Virology 68: 426–439. - PubMed
-
- Lazarowitz SG, Choppin PW (1975) Enhancement of the infectivity of influenza A and B viruses by proteolytic cleavage of the hemagglutinin polypeptide. Virology 68: 440–454. - PubMed
-
- Kido H, Yokogoshi Y, Sakai K, Tashiro M, Kishino Y, et al. (1992) Isolation and characterization of a novel trypsin-like protease found in rat bronchiolar epithelial Clara cells. A possible activator of the viral fusion glycoprotein. J Biol Chem 267: 13573–13579. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials

