Enterohemorrhagic Escherichia coli hemolysin employs outer membrane vesicles to target mitochondria and cause endothelial and epithelial apoptosis
- PMID: 24348251
- PMCID: PMC3861543
- DOI: 10.1371/journal.ppat.1003797
Enterohemorrhagic Escherichia coli hemolysin employs outer membrane vesicles to target mitochondria and cause endothelial and epithelial apoptosis
Abstract
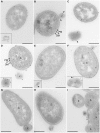
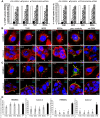
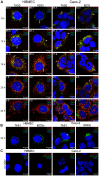
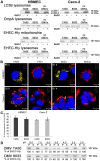
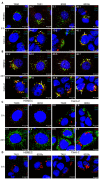
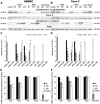
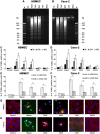
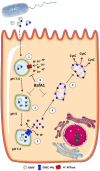
Enterohemorrhagic Escherichia coli (EHEC) strains cause diarrhea and hemolytic uremic syndrome resulting from toxin-mediated microvascular endothelial injury. EHEC hemolysin (EHEC-Hly), a member of the RTX (repeats-in-toxin) family, is an EHEC virulence factor of increasingly recognized importance. The toxin exists as free EHEC-Hly and as EHEC-Hly associated with outer membrane vesicles (OMVs) released by EHEC during growth. Whereas the free toxin is lytic towards human endothelium, the biological effects of the OMV-associated EHEC-Hly on microvascular endothelial and intestinal epithelial cells, which are the major targets during EHEC infection, are unknown. Using microscopic, biochemical, flow cytometry and functional analyses of human brain microvascular endothelial cells (HBMEC) and Caco-2 cells we demonstrate that OMV-associated EHEC-Hly does not lyse the target cells but triggers their apoptosis. The OMV-associated toxin is internalized by HBMEC and Caco-2 cells via dynamin-dependent endocytosis of OMVs and trafficked with OMVs into endo-lysosomal compartments. Upon endosome acidification and subsequent pH drop, EHEC-Hly is separated from OMVs, escapes from the lysosomes, most probably via its pore-forming activity, and targets mitochondria. This results in decrease of the mitochondrial transmembrane potential and translocation of cytochrome c to the cytosol, indicating EHEC-Hly-mediated permeabilization of the mitochondrial membranes. Subsequent activation of caspase-9 and caspase-3 leads to apoptotic cell death as evidenced by DNA fragmentation and chromatin condensation in the intoxicated cells. The ability of OMV-associated EHEC-Hly to trigger the mitochondrial apoptotic pathway in human microvascular endothelial and intestinal epithelial cells indicates a novel mechanism of EHEC-Hly involvement in the pathogenesis of EHEC diseases. The OMV-mediated intracellular delivery represents a newly recognized mechanism for a bacterial toxin to enter host cells in order to target mitochondria.
Conflict of interest statement
The authors have declared that no competing interests exist.
Figures






References
-
- Tarr PI, Gordon CA, Chandler WL (2005) Shiga-toxin-producing Escherichia coli and haemolytic uraemic syndrome. Lancet 365: 1073–1086. - PubMed
-
- Zoja C, Buelli S, Morigi M (2010) Shiga toxin-associated hemolytic uremic syndrome: pathophysiology of endothelial dysfunction. Pediatr Nephrol 25: 2231–2240. - PubMed
-
- Bielaszewska M, Bauwens A, Greune L, Kemper B, Dobrindt U, et al. (2009) Vacuolisation of human microvascular endothelial cells by enterohaemorrhagic Escherichia coli . Thromb Haemost 102: 1080–1092. - PubMed
Publication types
MeSH terms
Substances
Associated data
- Actions
- Actions
- Actions
- Actions
- Actions
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials

