Placental syncytium forms a biophysical barrier against pathogen invasion
- PMID: 24348256
- PMCID: PMC3861541
- DOI: 10.1371/journal.ppat.1003821
Placental syncytium forms a biophysical barrier against pathogen invasion
Abstract
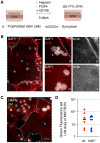
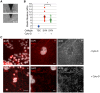
Fetal syncytiotrophoblasts form a unique fused multinuclear surface that is bathed in maternal blood, and constitutes the main interface between fetus and mother. Syncytiotrophoblasts are exposed to pathogens circulating in maternal blood, and appear to have unique resistance mechanisms against microbial invasion. These are due in part to the lack of intercellular junctions and their receptors, the Achilles heel of polarized mononuclear epithelia. However, the syncytium is immune to receptor-independent invasion as well, suggesting additional general defense mechanisms against infection. The difficulty of maintaining and manipulating primary human syncytiotrophoblasts in culture makes it challenging to investigate the cellular and molecular basis of host defenses in this unique tissue. Here we present a novel system to study placental pathogenesis using murine trophoblast stem cells (mTSC) that can be differentiated into syncytiotrophoblasts and recapitulate human placental syncytium. Consistent with previous results in primary human organ cultures, murine syncytiotrophoblasts were found to be resistant to infection with Listeria monocytogenes via direct invasion and cell-to-cell spread. Atomic force microscopy of murine syncytiotrophoblasts demonstrated that these cells have a greater elastic modulus than mononuclear trophoblasts. Disruption of the unusually dense actin structure--a diffuse meshwork of microfilaments--with Cytochalasin D led to a decrease in its elastic modulus by 25%. This correlated with a small but significant increase in invasion of L. monocytogenes into murine and human syncytium. These results suggest that the syncytial actin cytoskeleton may form a general barrier against pathogen entry in humans and mice. Moreover, murine TSCs are a genetically tractable model system for the investigation of specific pathways in syncytial host defenses.
Conflict of interest statement
The authors have declared that no competing interests exist.
Figures



References
-
- Goldenberg RL, Hauth JC, Andrews WW (2000) Intrauterine infection and preterm delivery. N Engl J Med 342: 1500–1507. - PubMed
-
- Pouillot R, Hoelzer K, Jackson KA, Henao OL, Silk BJ (2012) Relative risk of listeriosis in Foodborne Diseases Active Surveillance Network (FoodNet) sites according to age, pregnancy, and ethnicity. Clin Infect Dis 54 (Suppl 5) S405–410. - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

