Genetic recombination is targeted towards gene promoter regions in dogs
- PMID: 24348265
- PMCID: PMC3861134
- DOI: 10.1371/journal.pgen.1003984
Genetic recombination is targeted towards gene promoter regions in dogs
Abstract
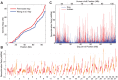
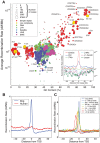
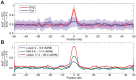
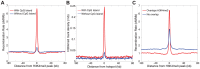
The identification of the H3K4 trimethylase, PRDM9, as the gene responsible for recombination hotspot localization has provided considerable insight into the mechanisms by which recombination is initiated in mammals. However, uniquely amongst mammals, canids appear to lack a functional version of PRDM9 and may therefore provide a model for understanding recombination that occurs in the absence of PRDM9, and thus how PRDM9 functions to shape the recombination landscape. We have constructed a fine-scale genetic map from patterns of linkage disequilibrium assessed using high-throughput sequence data from 51 free-ranging dogs, Canis lupus familiaris. While broad-scale properties of recombination appear similar to other mammalian species, our fine-scale estimates indicate that canine highly elevated recombination rates are observed in the vicinity of CpG rich regions including gene promoter regions, but show little association with H3K4 trimethylation marks identified in spermatocytes. By comparison to genomic data from the Andean fox, Lycalopex culpaeus, we show that biased gene conversion is a plausible mechanism by which the high CpG content of the dog genome could have occurred.
Conflict of interest statement
The authors have declared that no competing interests exist.
Figures
References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

