The NuRD chromatin-remodeling enzyme CHD4 promotes embryonic vascular integrity by transcriptionally regulating extracellular matrix proteolysis
- PMID: 24348274
- PMCID: PMC3861115
- DOI: 10.1371/journal.pgen.1004031
The NuRD chromatin-remodeling enzyme CHD4 promotes embryonic vascular integrity by transcriptionally regulating extracellular matrix proteolysis
Abstract
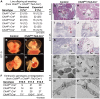
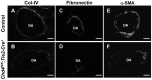
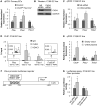
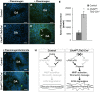
The extracellular matrix (ECM) supports vascular integrity during embryonic development. Proteolytic degradation of ECM components is required for angiogenesis, but excessive ECM proteolysis causes blood vessel fragility and hemorrhage. Little is understood about how ECM proteolysis is transcriptionally regulated during embryonic vascular development. We now show that the NuRD ATP-dependent chromatin-remodeling complex promotes vascular integrity by preventing excessive ECM proteolysis in vivo. Mice lacking endothelial CHD4--a catalytic subunit of NuRD complexes--died at midgestation from vascular rupture. ECM components surrounding rupture-prone vessels in Chd4 mutants were significantly downregulated prior to embryonic lethality. Using qPCR arrays, we found two critical mediators of ECM stability misregulated in mutant endothelial cells: the urokinase-type plasminogen activator receptor (uPAR or Plaur) was upregulated, and thrombospondin-1 (Thbs1) was downregulated. Chromatin immunoprecipitation assays showed that CHD4-containing NuRD complexes directly bound the promoters of these genes in endothelial cells. uPAR and THBS1 respectively promote and inhibit activation of the potent ECM protease plasmin, and we detected increased plasmin activity around rupture-prone vessels in Chd4 mutants. We rescued ECM components and vascular rupture in Chd4 mutants by genetically reducing urokinase (uPA or Plau), which cooperates with uPAR to activate plasmin. Our findings provide a novel mechanism by which a chromatin-remodeling enzyme regulates ECM stability to maintain vascular integrity during embryonic development.
Conflict of interest statement
The authors have declared that no competing interests exist.
Figures
Similar articles
-
CHD4-regulated plasmin activation impacts lymphovenous hemostasis and hepatic vascular integrity.J Clin Invest. 2016 Jun 1;126(6):2254-66. doi: 10.1172/JCI84652. Epub 2016 May 3. J Clin Invest. 2016. PMID: 27140400 Free PMC article.
-
The NuRD chromatin-remodeling complex enzyme CHD4 prevents hypoxia-induced endothelial Ripk3 transcription and murine embryonic vascular rupture.Cell Death Differ. 2020 Feb;27(2):618-631. doi: 10.1038/s41418-019-0376-8. Epub 2019 Jun 24. Cell Death Differ. 2020. PMID: 31235857 Free PMC article.
-
Endothelial Chromatin-Remodeling Enzymes Regulate the Production of Critical ECM Components During Murine Lung Development.Arterioscler Thromb Vasc Biol. 2024 Aug;44(8):1784-1798. doi: 10.1161/ATVBAHA.124.320881. Epub 2024 Jun 13. Arterioscler Thromb Vasc Biol. 2024. PMID: 38868942 Free PMC article.
-
Role of uPA/uPAR in the modulation of angiogenesis.Chem Immunol Allergy. 2014;99:105-22. doi: 10.1159/000353310. Epub 2013 Oct 17. Chem Immunol Allergy. 2014. PMID: 24217605 Review.
-
Chromatin Remodulator CHD4: A Potential Target for Cancer Interception.Genes (Basel). 2025 Feb 15;16(2):225. doi: 10.3390/genes16020225. Genes (Basel). 2025. PMID: 40004553 Free PMC article. Review.
Cited by
-
CHD4-regulated plasmin activation impacts lymphovenous hemostasis and hepatic vascular integrity.J Clin Invest. 2016 Jun 1;126(6):2254-66. doi: 10.1172/JCI84652. Epub 2016 May 3. J Clin Invest. 2016. PMID: 27140400 Free PMC article.
-
Covalent Modifications of Histone H3K9 Promote Binding of CHD3.Cell Rep. 2017 Oct 10;21(2):455-466. doi: 10.1016/j.celrep.2017.09.054. Cell Rep. 2017. PMID: 29020631 Free PMC article.
-
A role for repressive complexes and H3K9 di-methylation in PRDM5-associated brittle cornea syndrome.Hum Mol Genet. 2015 Dec 1;24(23):6565-79. doi: 10.1093/hmg/ddv345. Epub 2015 Sep 22. Hum Mol Genet. 2015. PMID: 26395458 Free PMC article.
-
Epigenetic roles of chromatin remodeling complexes in bone biology and the pathogenesis of bone‑related disease (Review).Int J Mol Med. 2025 Aug;56(2):115. doi: 10.3892/ijmm.2025.5556. Epub 2025 May 30. Int J Mol Med. 2025. PMID: 40444490 Free PMC article. Review.
-
Sequencing Overview of Ewing Sarcoma: A Journey across Genomic, Epigenomic and Transcriptomic Landscapes.Int J Mol Sci. 2015 Jul 16;16(7):16176-215. doi: 10.3390/ijms160716176. Int J Mol Sci. 2015. PMID: 26193259 Free PMC article. Review.
References
-
- Davis GE, Senger DR (2005) Endothelial extracellular matrix: biosynthesis, remodeling, and functions during vascular morphogenesis and neovessel stabilization. Circ Res 97: 1093–1107. - PubMed
-
- Sakalihasan N, Limet R, Defawe OD (2005) Abdominal aortic aneurysm. Lancet 365: 1577–1589. - PubMed
-
- Ruigrok YM, Rinkel GJ, Wijmenga C (2005) Genetics of intracranial aneurysms. Lancet Neurol 4: 179–189. - PubMed
-
- Poschl E, Schlotzer-Schrehardt U, Brachvogel B, Saito K, Ninomiya Y, et al. (2004) Collagen IV is essential for basement membrane stability but dispensable for initiation of its assembly during early development. Development 131: 1619–1628. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Miscellaneous

