Stress modulates intestinal secretory immunoglobulin A
- PMID: 24348350
- PMCID: PMC3845795
- DOI: 10.3389/fnint.2013.00086
Stress modulates intestinal secretory immunoglobulin A
Abstract
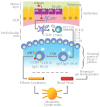
Stress is a response of the central nervous system to environmental stimuli perceived as a threat to homeostasis. The stress response triggers the generation of neurotransmitters and hormones from the hypothalamus pituitary adrenal axis, sympathetic axis and brain gut axis, and in this way modulates the intestinal immune system. The effects of psychological stress on intestinal immunity have been investigated mostly with the restraint/immobilization rodent model, resulting in an up or down modulation of SIgA levels depending on the intensity and time of exposure to stress. SIgA is a protein complex formed by dimeric (dIgA) or polymeric IgA (pIgA) and the secretory component (SC), a peptide derived from the polymeric immunoglobulin receptor (pIgR). The latter receptor is a transmembrane protein expressed on the basolateral side of gut epithelial cells, where it uptakes dIgA or pIgA released by plasma cells in the lamina propria. As a result, the IgA-pIgR complex is formed and transported by vesicles to the apical side of epithelial cells. pIgR is then cleaved to release SIgA into the luminal secretions of gut. Down modulation of SIgA associated with stress can have negative repercussions on intestinal function and integrity. This can take the form of increased adhesion of pathogenic agents to the intestinal epithelium and/or an altered balance of inflammation leading to greater intestinal permeability. Most studies on the molecular and biochemical mechanisms involved in the stress response have focused on systemic immunity. The present review analyzes the impact of stress (mostly by restraint/immobilization, but also with mention of other models) on the generation of SIgA, pIgR and other humoral and cellular components involved in the intestinal immune response. Insights into these mechanisms could lead to better therapies for protecting against pathogenic agents and avoiding epithelial tissue damage by modulating intestinal inflammation.
Keywords: SIgA; brain-gut axis; glucocorticoids; intestinal mucosa; pIgR; restraint-stress.
Figures



References
Publication types
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous

