Oscillatory interaction between amygdala and hippocampus coordinates behavioral modulation based on reward expectation
- PMID: 24348352
- PMCID: PMC3847563
- DOI: 10.3389/fnbeh.2013.00177
Oscillatory interaction between amygdala and hippocampus coordinates behavioral modulation based on reward expectation
Abstract
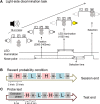
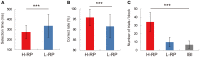
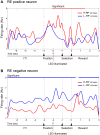
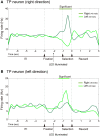
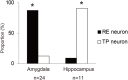
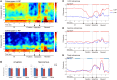
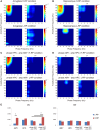
The aim of this study is to examine how the amygdala and hippocampus interact for behavioral performance modulated by different Reward-expectations (REs). We simultaneously recorded neuronal spikes and local field potential from the basolateral amygdala and hippocampal CA1 while rats were performing a light-side discrimination task with different expectations of a high or low probability of reward delivery. Here, we report the following results. First, the rats actually modulated their behavioral performance on their expectations of a high or low probability of reward. Second, we found more neurons related to RE in the amygdala and more neurons related to task performance in the hippocampus. Third, a prominent increase in the coherence of high-frequency oscillations (HFOs) (90-150 Hz) between the amygdala and the hippocampus was present during high RE. Fourth, coherent HFOs during inter-trial intervals and theta coherence during trials had significant correlations with the behavioral goal-selection time. Finally, cross-frequency couplings of LFPs within and across the amygdala and hippocampus occurred during ITI. These results suggest that the amygdala and hippocampus have different functional roles in the present task with different REs, and the distinctive band of coherence between the amygdala and the hippocampus contributes to behavioral modulation on the basis of REs. We propose that the amygdala influences firing rates and the strength of synchronization of hippocampal neurons through coherent oscillation, which is a part of the mechanism of how reward expectations modulate goal-directed behavior.
Keywords: amygdala; behavioral modulation; hippocampus; oscillation synchrony; reward expectation.
Figures
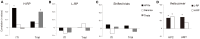
References
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

