The functional anatomy of attention: a DCM study
- PMID: 24348359
- PMCID: PMC3845206
- DOI: 10.3389/fnhum.2013.00784
The functional anatomy of attention: a DCM study
Abstract
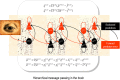
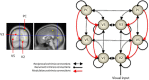
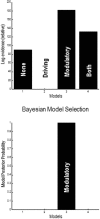
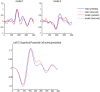
Recent formulations of attention-in terms of predictive coding-associate attentional gain with the expected precision of sensory information. Formal models of the Posner paradigm suggest that validity effects can be explained in a principled (Bayes optimal) fashion in terms of a cue-dependent setting of precision or gain on the sensory channels reporting anticipated target locations, which is updated selectively by invalid targets. This normative model is equipped with a biologically plausible process theory in the form of predictive coding, where precision is encoded by the gain of superficial pyramidal cells reporting prediction error. We used dynamic causal modeling to assess the evidence in magnetoencephalographic responses for cue-dependent and top-down updating of superficial pyramidal cell gain. Bayesian model comparison suggested that it is almost certain that differences in superficial pyramidal cells gain-and its top-down modulation-contribute to observed responses; and we could be more than 80% certain that anticipatory effects on post-synaptic gain are limited to visual (extrastriate) sources. These empirical results speak to the role of attention in optimizing perceptual inference and its formulation in terms of predictive coding.
Keywords: Posner; active inference; attention; cortical gain control; precision; predictive coding.
Figures


References
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

