Gimme shelter: how Vibrio fischeri successfully navigates an animal's multiple environments
- PMID: 24348467
- PMCID: PMC3843225
- DOI: 10.3389/fmicb.2013.00356
Gimme shelter: how Vibrio fischeri successfully navigates an animal's multiple environments
Abstract
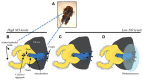
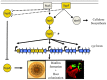
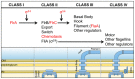
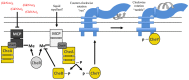
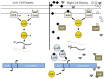
Bacteria successfully colonize distinct niches because they can sense and appropriately respond to a variety of environmental signals. Of particular interest is how a bacterium negotiates the multiple, complex environments posed during successful infection of an animal host. One tractable model system to study how a bacterium manages a host's multiple environments is the symbiotic relationship between the marine bacterium, Vibrio fischeri, and its squid host, Euprymna scolopes. V. fischeri encounters many different host surroundings ranging from initial contact with the squid to ultimate colonization of a specialized organ known as the light organ. For example, upon recognition of the squid, V. fischeri forms a biofilm aggregate outside the light organ that is required for efficient colonization. The bacteria then disperse from this biofilm to enter the organ, where they are exposed to nitric oxide, a molecule that can act as both a signal and an antimicrobial. After successfully managing this potentially hostile environment, V. fischeri cells finally establish their niche in the deep crypts of the light organ where the bacteria bioluminesce in a pheromone-dependent fashion, a phenotype that E. scolopes utilizes for anti-predation purposes. The mechanism by which V. fischeri manages these environments to outcompete all other bacterial species for colonization of E. scolopes is an important and intriguing question that will permit valuable insights into how a bacterium successfully associates with a host. This review focuses on specific molecular pathways that allow V. fischeri to establish this exquisite bacteria-host interaction.
Keywords: Euprymna scolopes; Vibrio fischeri; antimicrobials; biofilm; bioluminescence; chemotaxis; symbiosis.
Figures

References
-
- Altura M. A., Heath-Heckman E. A., Gillette A., Kremer N., Krachler A. M., Brennan C., et al. (2013). The first engagement of partners in the Euprymna scolopes-Vibrio fischeri symbiosis is a two-step process initiated by a few environmental symbiont cells. Environ. Microbiol. 15 2937–2950 10.1111/1462-2920.12179 - DOI - PMC - PubMed
-
- Baudouin E., Pauly N., Puppo A. (2007). “Nitric oxide in nitrogen-fixing symbiosis,” in Nitric Oxide in Plant Growth, Development, and Stress Physiology, eds Lamattina L., Polacco J. (Berlin: Springer; ) 173–186
Publication types
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

