Stem cells supporting other stem cells
- PMID: 24348512
- PMCID: PMC3847370
- DOI: 10.3389/fgene.2013.00257
Stem cells supporting other stem cells
Abstract
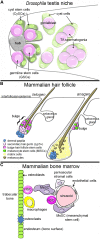
Adult stem cell therapies are increasingly prevalent for the treatment of damaged or diseased tissues, but most of the improvements observed to date are attributed to the ability of stem cells to produce paracrine factors that have a trophic effect on existing tissue cells, improving their functional capacity. It is now clear that this ability to produce trophic factors is a normal and necessary function for some stem cell populations. In vivo adult stem cells are thought to self-renew due to local signals from the microenvironment where they live, the niche. Several niches have now been identified which harbor multiple stem cell populations. In three of these niches - the Drosophila testis, the bulge of the mammalian hair follicle, and the mammalian bone marrow - one type of stem cell has been found to produce factors that contribute to the maintenance of a second stem cell population in the shared niche. In this review, I will examine the architecture of these three niches and discuss the molecular signals involved. Together, these examples establish a new paradigm for stem cell behavior, that stem cells can promote the maintenance of other stem cells.
Keywords: Drosophila stem cells; germline stem cell; hair follicle stem cell; hematopoietic stem cells; self-renewal pathways; stem cell niche; stem cell therapy.
Figures
Similar articles
-
[Regulation of germline stem cells: the niche expands in Drosophila].Med Sci (Paris). 2007 Jun-Jul;23(6-7):611-8. doi: 10.1051/medsci/20072367611. Med Sci (Paris). 2007. PMID: 17631836 Review. French.
-
Haematopoietic stem cell niche in Drosophila.Bioessays. 2007 Aug;29(8):713-6. doi: 10.1002/bies.20613. Bioessays. 2007. PMID: 17620327 Review.
-
Stem cell niche: structure and function.Annu Rev Cell Dev Biol. 2005;21:605-31. doi: 10.1146/annurev.cellbio.21.012704.131525. Annu Rev Cell Dev Biol. 2005. PMID: 16212509 Review.
-
The stem cell niche: lessons from the Drosophila testis.Development. 2011 Jul;138(14):2861-9. doi: 10.1242/dev.056242. Development. 2011. PMID: 21693509 Free PMC article. Review.
-
Stem cell niches in mammals.Exp Cell Res. 2007 Oct 1;313(16):3377-85. doi: 10.1016/j.yexcr.2007.07.027. Epub 2007 Aug 2. Exp Cell Res. 2007. PMID: 17764674 Review.
Cited by
-
The Dlg Module and Clathrin-Mediated Endocytosis Regulate EGFR Signaling and Cyst Cell-Germline Coordination in the Drosophila Testis.Stem Cell Reports. 2019 May 14;12(5):1024-1040. doi: 10.1016/j.stemcr.2019.03.008. Epub 2019 Apr 18. Stem Cell Reports. 2019. PMID: 31006632 Free PMC article.
-
Potential spermatogenesis recovery with bone marrow mesenchymal stem cells in an azoospermic rat model.Int J Mol Sci. 2014 Jul 24;15(8):13151-65. doi: 10.3390/ijms150813151. Int J Mol Sci. 2014. PMID: 25062349 Free PMC article.
-
The Drosophila histone methyl-transferase SET1 coordinates multiple signaling pathways in regulating male germline stem cell maintenance and differentiation.bioRxiv [Preprint]. 2024 Feb 14:2024.02.14.580277. doi: 10.1101/2024.02.14.580277. bioRxiv. 2024. Update in: Development. 2024 Aug 1;151(15):dev202729. doi: 10.1242/dev.202729. PMID: 38405894 Free PMC article. Updated. Preprint.
-
The Regenerative Effect of Bone Marrow-Derived Stem Cells in Spermatogenesis of Infertile Hamster.World J Plast Surg. 2017 Jan;6(1):18-25. World J Plast Surg. 2017. PMID: 28289609 Free PMC article.
-
Purinergic signaling: a common pathway for neural and mesenchymal stem cell maintenance and differentiation.Front Cell Neurosci. 2015 Jun 2;9:211. doi: 10.3389/fncel.2015.00211. eCollection 2015. Front Cell Neurosci. 2015. PMID: 26082684 Free PMC article. Review.
References
-
- Calvi L. M., Bromberg O., Rhee Y., Weber J. M., Smith J. N., Basil M. J., et al. (2012). Osteoblastic expansion induced by parathyroid hormone receptor signaling in murine osteocytes is not sufficient to increase hematopoietic stem cells. Blood 119 2489–2499 10.1182/blood-2011-06-360933 - DOI - PMC - PubMed
Publication types
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

